
Weak Acids
Introduction Acids which does not completely dissociate are termed weak acids. The conjugate base of these acids is very unstable due to which they tend […]

Introduction Acids which does not completely dissociate are termed weak acids. The conjugate base of these acids is very unstable due to which they tend […]
Introduction This article will tell us about the difference between the two types of cells: Electrolytic cells and Galvanic cells. Cells They are devices that […]

Introduction Strong acids are substances that on dissociation release a vast number of hydronium cations ( H+or H3O+). Strong acids also have a large value […]

1. Weak electrolyte definition Weak electrolyte are those electrolytes that have a low degree of dissociation at higher concentrations and hence their conductivity increases with […]
Know in one minute about Electrolytic cells Electrolytic cells are a type of electrochemical cell that uses electricity to initiate chemical reactions. It consists of […]

Introduction Minerals are natural substances that are extracted from rocks found either on the surface or inside the earth. Based on their natural properties, they […]
Introduction Strong Acids and Bases are those acids and bases which completely ionized into ions (H+ and OH- ions) in aqueous solutions. Strong Acids Before […]
Some readers might be in doubt that why Acetic acid is a weak electrolyte. In this section of the article, we will discuss the same […]
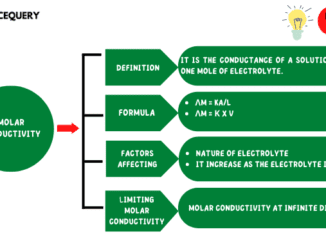
Know in one minute about molar conductivity Molar conductivity refers to the conducting power of ions in a solution. Its unit is S.m2.mol-1 (Siemens X […]
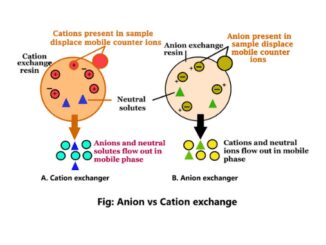
Introduction Anion exchange vs cation exchange is the two forms of ion-exchange chromatography. These are the two chemical methods used to separate molecules according to […]
Copyright © 2025 | WordPress Theme by MH Themes