Introduction
This article will tell us about the difference between the two types of cells: Electrolytic cells and Galvanic cells.
Cells
They are devices that either produce electricity or in chemistry, it is basically a setup or arrangement in which chemical reactions take place.
Based on this difference cells are divided into two types :
- Electrochemical cell
- Electrolytic cell
Generally when two or more cells are connected they form a battery
Let us now discuss these types of batteries in detail and also know about the difference between them along with the difference between Electrolytic cells and Galvanic cells.
Definition
Electrochemical Cells
An electrochemical cell is a device in which the chemical energy of the redox reaction is converted into electrical energy.
Batteries and fuel cells convert chemical energy into electrical energy and are used in various instruments and devices. The reactions carried out electrochemically can be energy efficient and less polluting. The most common cell is a Galvanic cell.
Galvanic Cells
Electrochemical cells which convert the chemical energy of a spontaneous redox reaction into electrical energy are called galvanic or voltaic cells. It is composed of two half-cells, each having different salt solutions, connected through an electric current.
Electrolytic Cells
Electrolytic cells are those cells in which electrical energy is used to carry out non-spontaneous chemical reactions and the reactions taking place in an electrolytic cell are called Electrolysis.
In an electrolytic cell, an external source of voltage is used to bring about a chemical reaction. The simplest electrolytic cells consist of two copper strips (which act as an anode and cathode) dipping in an aqueous solution of copper sulfate.
When a DC voltage is applied to the two electrodes, copper is dissolved (oxidized) at the anode and deposited (reduced) at the cathode, and the following reactions occur.
At cathode, Cu2+ (aq) + 2e-→ Cu(s)
At anode, Cu(s) → Cu2+ (s) + 2e-
Structure And Function of the electrolytic cell
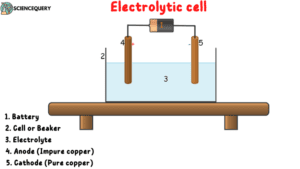
As we know that Electrolytic cell converts electrical energy into chemical energy, hence the electricity provided to the electrodes converts into ions. Hence it is a circuit involving
Multiple components are essential for the release of ions.
Therefore, the components which constitutes the circuit for electrolytic cell are
1. Battery
The battery plays an important role in the circuit of an electrochemical cell, it is the source of electrical energy in the whole arrangement.
Electrons are released from it which then go to the negatively charged electrode known as the cathode. Then the metal ion is formed at the cathode.
2. Container Cell
It is the holder or the place at which the reactions, complete mechanism, and electrolytic cell are assembled.
It is generally a beaker or a container. All other components of an electrolytic cell are present inside it except the battery.
3. Electrolyte
An electrolyte is an aqueous solution that has the property to conduct electricity. It acts as a medium for the reactions commencing on the anode as well as the cathode.
4. Electrodes
Are rods or a material made of either metal or non-metal. Electrodes either dissolve or becomes the spot for the accumulation of other metals.
Depending upon the reduction potential of each material, its corresponding electrode becomes an anode or cathode.
- Anode: For an electrolytic cell the electrode at which oxidation takes place is known as Anode. At the anode, electrons are released and the electrode starts to dissolve. For example, in the industrial process, an impure copper rod is made as an anode that dissolves on passing current.
- Cathode: Similarly when an electrode reduction takes place inside an electrolytic cell is considered a cathode. At the cathode, the ions gain electrons and form the crystals of the respective material and hence the solid crystals of the material get deposited in that electrode. For example, In industries, the pure copper rod is made cathode at which copper ions are deposited.
The function of an electrolytic cell
Since the process taking place in an electrolytic cell is electrolysis, we can use it to produce or obtain many products from it for example,
1. Extraction of metals
It can be used to extract metals from its ores, and this process is known as Electrometallurgy. Metals that can be extracted are zinc ( which is extracted from its ore), Aluminium (extracted from cryolite and bauxite), Magnesium
2. Refining of metals
Process of removing impurities in order to make the metal pure.
3. Manufacturing of Chemicals
When an electric current is passed through the compound solution it breaks down to form other chemicals.
4. Electro-Deposition
It is the process of depositing metal over another dissimilar metal or any non-metal.
5. Electroplating
It is similar to the Electro-deposition process, except it is used to protect any surface while the Electro-deposition process is mostly used for decorative purposes.
6. Electro-deposition of rubber
The rubber extracted from trees consists of very fine colloidal particles which are negatively charged. These particles on electrolysis move towards the anode and get deposited on it.
7. Electrometallization
deposition of metal over a conducting surface is Electrometallization.
8. Electro-Facing
Harder metals are coated over any other metallic surface to increase durability.
Structure and Function of Galvanic Cell
A galvanic cell generally looks like this, and over here it is specifically a daniel cell with zinc and copper electrodes.
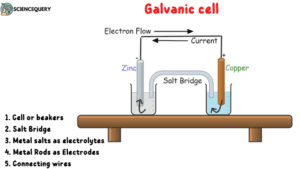
The cell which converts chemical energy into electrical energy is a Galvanic cell or the circuit which uses a set of chemical reactions to form electrical energy.
Therefore the cell contains a circuit that is capable of producing electricity.
-
Cell or beakers
These are the platform for the apparatus of a Galvanic cell.
These hold the electrodes, salt bridge, and electrolytes in them. Unlike in electrolytic cells, they are separate for both electrodes. Each cell is also referred to as a half-cell.
-
Electrolytes
These are electricity-conducting aqueous solutions. In the case of galvanic cells, the electrolytes are the metal salts of the corresponding metal in the electrodes.
For example- The zinc rod is dipped in ZnSO4 while the Copper rod is dipped in CuSO4 solution.
-
Electrodes
In the case of Galvanic cells electrodes are rods or sometimes sheets made of pure metal, there are two electrodes, both of which are made up of dissimilar metals or a single metal with different concentrations. Like electrolytic cells, Galvanic cells too have an electrode working as Anode while the other is a cathode.
1. Anode: The electrode at which electrons are released or oxidation takes place is known as Anode. In the Galvanic cell, the anode acts as a Negative terminal.
2. Cathode: The electrode at which electrons are absorbed or the half of the cell at which reduction takes place is known as a Cathode. It acts as a positive terminal in a galvanic cell.
-
Salt Bridge
It is an internal connection between the two half-cells of a Galvanic cell. The salt bridge is generally a tube filled with a metal salt which is different from both the electrolytes. The function of a salt bridge is to prevent diffusion or mechanical flow between the electrolytes.
Functions of Galvanic Cell
1. For making fuel cells
Fuel cells are storage devices that consist of electricity as a fuel, or they are simply, batteries assembled on a larger scale.
2. Manufacturing of small-scale batteries
These are also used in making small scaled batteries which are used in day-to-day life.
3. Modern-day mobile batteries
The batteries in modern-day mobile phones also act as a galvanic cells when they are in the discharging state or when the battery is in use and unplugged from the charger.
Difference Between Electrolytic and Galvanic Cell
Electrolytic Cell |
Galvanic Cell |
| Converts electrical energy to spontaneous chemical reactions. | Converts Chemical energy to electrical energy. |
| Consists of one electrolyte. | Consist of electrolytes in the form of salts of metals used in making electrodes. Here the number can be more than one. |
| Has only one cell. | Has separate cells for each electrode, known as half cells. |
| The cathode is generally negative, while the anode is at a positive terminal. | Here, the anode is the negative terminal while the cathode is the positive terminal. |
| Here an external power source is connected. | No external power is connected as it generates electricity by itself. |
| Used for extracting metals. | Used in making batteries. |
Q&A
1. What is the difference between a galvanic cell and an electrolytic cell?
The galvanic cell generates electricity while the electrolytic cell uses electricity to complete chemical reactions.
2. What is the difference between a galvanic cell (such as a Daniell cell) and an electrolytic cell?
A galvanic cell has a separate beaker or cell known as a half cell for each electrode, while an electrolytic cell has only one cell for the whole arrangement.
3. What is the difference between an electrolytic cell and a galvanic/voltaic cell?
It consists of a salt bridge to prevent diffusion while electrolytic cell does not have any.
4. What is the difference between electrolytic and galvanic cells?
Galvanic does not need any external power to initiate its process while electrolytic cell requires electricity to complete the whole process.
5. What is the difference between galvanic and electrolytic cells?
A galvanic cell is used in making batteries while the electrolytic cell is used in a process that can purify or extract or coat a metal surface other.
Closing Summary
Cells are of two types
- Electrochemical cell
- Electrolytic cell
An electrochemical cell is a device in which the chemical energy of the redox reaction is converted into electrical energy.
Electrolytic cells are those cells in which electrical energy is used to carry out non-spontaneous chemical reactions.
The components of electrolytic cells are
- Battery
- Cell or Beaker
- Electrolyte
- Anode
- Cathode
Its functions include
-
- Electrometallurgy
- Refining of metals
- Manufacturing of Chemicals
- Electro-Deposition
- Electroplating
Electrochemical cells that convert the chemical energy of a spontaneous redox reaction into electrical energy are called galvanic or voltaic cells.
The cell has the following components:
- Cell or beakers
- Salt Bridge
- Metal salts as electrolytes
- Metal Rods as Electrodes
- Connecting wires
Functions of Galvanic cell
-
- Used for making fuel cells
- Making small scaled batteries
- Batteries of mobile phones
Written By: Bharat Awasthi
