
Is Bronze Magnetic?
Is Bronze Magnetic? Know in one minute Bronze is an alloy made up of copper and tin mixed with each other, containing Tin with a […]

Is Bronze Magnetic? Know in one minute Bronze is an alloy made up of copper and tin mixed with each other, containing Tin with a […]
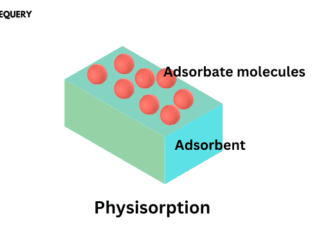
Know about Physisorption in one minute Physisorption is a type of adsorption process that involves weak van der Waals forces between the adsorbate and the […]
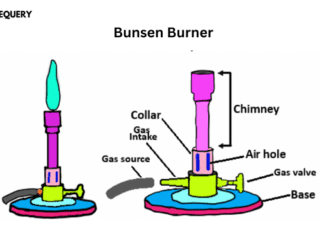
Know in one minute about the Bunsen burner The Bunsen burner is a high-temperature heating device. Widely used in laboratories all over the world for […]

What are lab grown diamonds: Know in one minute Lab-grown diamonds are diamonds that are produced in a laboratory and possess the same physical, chemical, […]
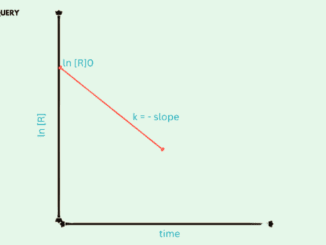
Know in one minute about First order reactions First order reaction is a type of chemical reaction in which the rate of the reaction depends […]
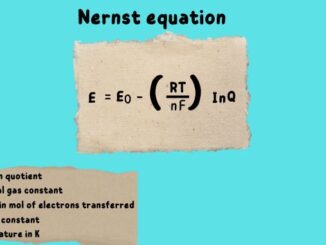
How to define the Nernst equation This equation was named after German scientist Walther Nernst. It is important in electrochemistry as it provides a relation […]
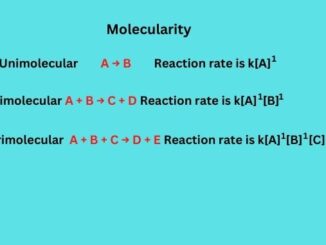
Know in one minute about molecularity Molecularity refers to the number of reactant molecules involved in an elementary reaction. Mathematically it is calculated by the […]

Know in one minute about Secondary battery The secondary battery also known as a rechargeable battery is a type of electrochemical battery that can be […]

Know in one minute about Primary battery Primary batteries are a type of electrochemical cell that cannot be reused when once depleted. They can also […]

Introduction Acids which does not completely dissociate are termed weak acids. The conjugate base of these acids is very unstable due to which they tend […]
Copyright © 2024 | WordPress Theme by MH Themes