Introduction
Anion exchange vs cation exchange is the two forms of ion-exchange chromatography. These are the two chemical methods used to separate molecules according to their net surface charge. There are some differences between these two chemical methods.
An ion is an atom or molecule. When an atom is charged positively or negatively by accepting or rejecting electrons, that atom is called an ion. One of the ways to separate these ions from a mixture is ion exchange chromatography. In ion-exchange chromatography, ions are exchanged in two ways. These are anion exchange and cation exchange.
A cation is a positively charged ion because it has more protons than electrons and anion is a negatively charged ion because it has more electrons than protons. These two ions easily attract each other and form ionic compounds. However the anion exchange and cation exchange methods are different from each other. It is described here (1) & (5).
Principal
1. Anion exchange
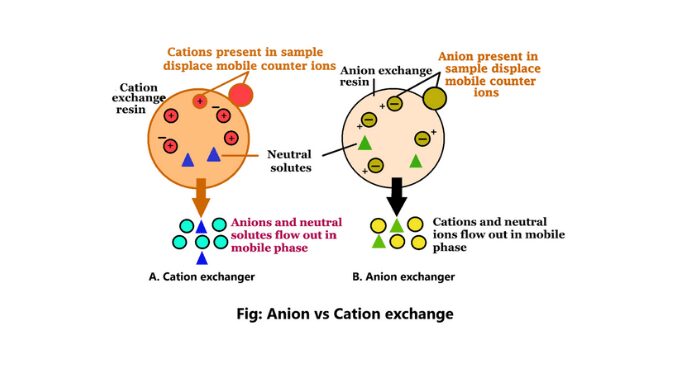
Anion exchange chromatography is a chemical method that separates the molecules according to the charges using an ion-exchange resin including positively charged groups. It is a biotechnical method. Anion exchangers in this process are positively charged exchangers (1).
Properties
1. Anion exchange is mainly used for the purification of amino acids, proteins, carbohydrates, etc.
2. This exchange method will bind the negatively charged molecules.
3. The anion exchange method can separate a large type of molecules
4. This is a chemical process that can bind an exchange resin and is reported as the equivalent of a single charged ion per gram of resin (2).
Principle
1. In the anion exchange method the charge on the net surface of a protein varies with the pH in a way that is determined by the isoelectric point or pI of the protein.
2. If the buffer pH level is raised above the pI of a protein, it also carries a net negative charge.
3. In the anion exchange method, if the pH is below the pI, the protein carries a net positive charge.
4. The positively charged ion exchange resin is chosen when the protein carries a net negative charge in the pH.
5. Different pI standard proteins will have different degrees of charge at a given pH and thus have different relationships for positively charged surface groups in anion exchange media.
6. Since the pI of a protein is determined by its primary amino acid sequence it can be calculated.
7. At an individual loading buffer pH, all properly charged proteins will bind to the resin.
8. The proteins in anion exchange chromatography will be arranged in a sequence depending on their net surface charge (2) & (4).
2. Cation exchange
Cation exchange is a method of ion exchange chromatography. This method is used to isolate the molecules according to their net charges. The cation exchange method is used for both preparatory and analytical purposes.
Properties
1. The cation exchanger in the cation exchange method is a negatively charged exchanger.
2. Cation exchange method is mainly used to purify large molecules such as proteins, amino acids, etc.
3. This exchange method will bind the positively charged molecules.
4. These exchangers contain positively charged counter ions.
5. The pH for this type of chromatography is less than pI.
Principle
1. Here the net surface charge of a protein varies with pH as determined by the isoelectric point or pI of the protein.
2. The pH value of a protein is equal to the pI of the protein. So the protein will carry no net charge.
3. In this ion exchange method, if the pH level of the buffer is raised above the pI of a protein, it also carries a net negative charge.
4. When the protein carries a net positive charge in the working pH, then a cation exchange method is chosen.
5. The pI of a protein is determined by its primary amino acid sequence.
6. A buffer is then chosen that ensures a net charge for the protein.
7. The proteins in cation exchange will be arranged in a sequence depending on their net surface charge.
8. Different proteins are bound to the cation exchange method with different strengths (3) & (5).
Function or uses
Both Ion exchange methods have been widely used for many biochemical mixtures such as proteins, amino acids, sugars, nucleic acids, etc. Some of these are described below.
Anion exchange chromatography functions
1. The main function of anion exchange chromatography is to purify proteins, amino acids, and some acidic solutions.
2. Anion exchange method is used to separate the complex mixture of 18 amino acids obtained by the acid hydrolysis of proteins.
3. This type of ion exchange method is mainly used to isolate the charged molecules.
4. Anion exchange has positively charged groups that will attract negatively charged anions.
5. This method uses some preparative and analytical processes.
6. Combined with optimized binding, this method can be used in some stages of biomolecule isolation, separation of analytes, removal of contaminants such as endotoxins, host cell proteins, etc.
7. The method is important for all types of buffer pH and ionic energy.
8. Anion exchange can help in identifying the ion exchange which is very effective for the treatment
9. This method is used in many branches of the industry. Most of these applications are used to measure and analyze residues in pharmaceuticals, including iodides, sulfates, phosphates, as well as potassium and sodium.
10. This process is a very important tool to exchange or separate contaminants in low concentrations (2) & (4).
Cation exchange chromatography functions
1. The cation exchange method has negatively charged groups that will attract positively charged anions. These are known as basic ion exchange materials.
2. This method is used to treat hyperkalemia by accelerating potassium loss through the gut.
3. Cation exchange processes accept or donate electrons to the solution and are used in the oxidation-reduction process.
4. These are used in medicine to remove a particular positively charged ion.
5. The cation exchange method is a powerful magnet that attracts and retains contaminated minerals from the source water produced by electromagnetic attraction.
6. In the case of water treatment, cation exchange is used to remove divalent ions related to alkalinity.
7. This method is mainly used to isolate molecules such as proteins, amino acids, etc.
8. When cation exchange is combined with water, it can exchange a certain type of active ion and an electric ion in water (3).
Anion exchange vs cation exchange
Anion exchange and cation exchange are two types of ion exchange chromatography. They are used to separate the molecules from the solutions. Both methods are important for the exchange of ions. However, there are some differences between these two forms of ion-exchange chromatography. Anion exchange vs cation exchange is discussed with the help of a table below.
Anion exchange |
Cation exchange |
| 1. Anion exchange is a chemical process that isolates the molecules according to the charges using an ion-exchange resin including positively charged groups. | Cation exchange is a chemical method that is used to separate the molecules based on their net charges. |
| 2. This type of ion exchange method has positively charged groups that will attract negatively charged anions. | The cation exchange method has negatively charged groups that will attract positively charged cations. |
| 3. The anion exchanger in this exchange method is a positively charged exchanger. | The cation exchanger in the cation exchange method is a negatively charged exchanger. |
| 4. The anion exchange process will bind the negatively charged molecules. | This exchange method will bind the positively charged molecules. |
| 5. This method is formed from nonmetal molecules. | A cation is formed from metal molecules. |
| 6. In the anion exchange method, the number of electrons is more than the number of protons. | In the cation exchange method, the number of electrons is less than the number of protons. |
| 7. The stationary phase in anion exchange is positively charged. | Here, the stationary phase is negatively charged. |
| 8. Anion exchange method attracts another set of minerals. | The cation exchange process attracts a certain set of minerals. |
| 9. If an anion exchange method is used at a pH of 7.5, all proteins that have a pI < 7.5 will carry a net negative charge. | If a cation exchange method is used at a pH of 7.5, all proteins that have a pI > 7.5 will carry a net positive charge. |
| 10. Anion exchange method is run at 0.5 to 1.5 pH units above the pI of the compound. | This method is run at 0.5 to 1.5 pH units below the pI of the compound (2) & (3). |
Q&A
1. When to use cation vs anion exchange?
Anion exchange and cation exchange are used to separate the ion from a sample. Basically when the stationary phase is positively charged and negatively charged molecules are charged to be attracted to the phase, then anion exchange method is used.
On the other hand, the cation exchange process is used when mainly the molecules are positively charged.
2. How do I remember cation vs anion exchange?
The negatively charged ions are known as anions and the positively charged ions are cations. That means anions have two n’s for negative charges and cations have a “t” just like the plus sign for positive charges.
The cation exchange method will bind the positively charged molecules and the anion exchange method will bind the negatively charged molecules. In this way, I remember cation exchange vs anion exchange.
3. When do you use anion exchange vs cation exchange?
- Anion is positively charged groups and the cation exchange method has negatively charged groups.
- The anion exchange will attract negatively charged anions. And Cation will attract positively charged cations.
- If it is stable at a pI value above the pH, the cation exchange method is used. On the other side, if it is stable at a pI value below the pH, then the anion exchange method can be used.
