What is the structure of the diamond?
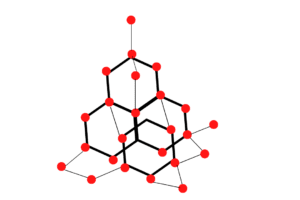
Diamond is an allotrope of carbon it is solid in nature. A solid is a state of matter where the molecules are in close arrangement with one another and have a specific structure. Besides diamonds, there are other allotropes of carbon namely graphite, fullerenes, and charcoal. Diamond is one of the hardest forms of carbon and is mainly used for making beautiful gemstones. The structure of the diamond is tetrahedral with each of the carbon atoms joined to the other four carbon atoms in a regular tetrahedral pattern.
These carbon atoms combined with the other four carbon atoms in a regular tetrahedral structure to form a rigid three-dimensional structure. So the structure of a diamond is like a 3D structure. Each carbon atom in a diamond is a covalent bond to the four surrounding carbon atoms (2) & (4).
How to draw the structure of the diamond?
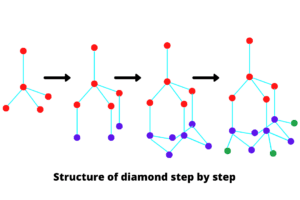
Each carbon atom of a diamond has four covalent bonds. These bonds are very strong. It takes high temperatures to provide the energy needed to break them down. For this reason, diamonds have a very high melting point. All the external electrons of each carbon atom are involved in covalent bonding.
A diamond has a specific structure. First, an atom is drawn in the center. And four more carbon atoms are drawn around it. Then the atoms are joined by a line. Four new carbon atoms are drawn parallel to the bottom layer. And with that atom, the newly drawn carbon atoms are connected by lines. Finally, four more carbon atoms are drawn bottom to them and connected by lines. In this way, the diamond structure is drawn.
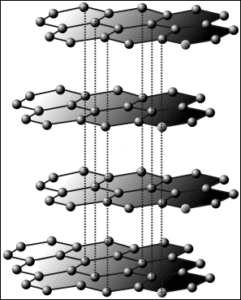
How does the crystalline structure of graphite compare with that of a diamond?
Structure of diamond |
Structure of graphite |
 |
|
Diamond and graphite are very interesting elements. Both are derived from carbon atoms. But they are completely different structurally. Both graphite and diamond are crystalline forms of carbon. However, there are some comparisons between graphite and diamond crystalline structures. In diamonds, the atoms are very closely packed. Each carbon atom in a diamond is a covalent bond to the four surrounding carbon atoms.
The carbon atoms in the diamond are joined to the other four carbon atoms in a regular tetrahedral arrangement. And giving a very strong and rigid three-dimensional structure. On the other side, in graphite, the carbon atoms are loosely packed. Each of the carbon atoms in graphite is bound to any three carbon atoms. The bond between these atoms is weak. Because in graphite the carbon atoms are thin sheets.
In graphite, the carbon atom is bonded to three carbon atoms only leaving one free valence. And giving a three-dimensional arrangement of hexagonal rings. So graphite and diamond are both allotropes of carbon, and there are some comparisons in their crystalline structure (1) & (4).
What is the crystalline structure of the diamond?
Crystal is a special form of solid. Particles of solids that are equipped with a specific rule are called crystalline. The molecules of this substance acquire a precise three-dimensional form. This type of substance is commonly called a crystalline substance. This particular type of microscopic arrangement of matter is called the crystalline structure. Diamond is a solid substance.
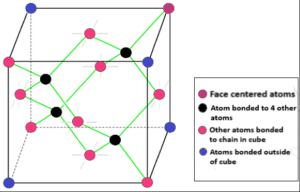
Diamond particles are arranged in a specific order to form a crystalline structure. The crystalline structure of the diamond is a face-centered cubic crystal structure or FCC. In this crystalline structure, each carbon atom is joined to four more carbon atoms in a regular tetrahedral pattern.
Diamond crystals can be arranged in a variety of shapes. The crystals of a diamond are formed like cubes, dodecahedra, and a combination of these shapes. The crystal of a diamond may not be completely smooth. But the crystal structure is triangular prisms. A diamond is a crystal of tetrahedrally bonded carbon atoms in a covalent lattice (spᶾ) that forms crystals in a diamond which is an alternative to the face-centered cubic structure. Each carbon atom is parallel to the other four carbon atoms (3) & (5).
Fig: Crystalline structure of diamond