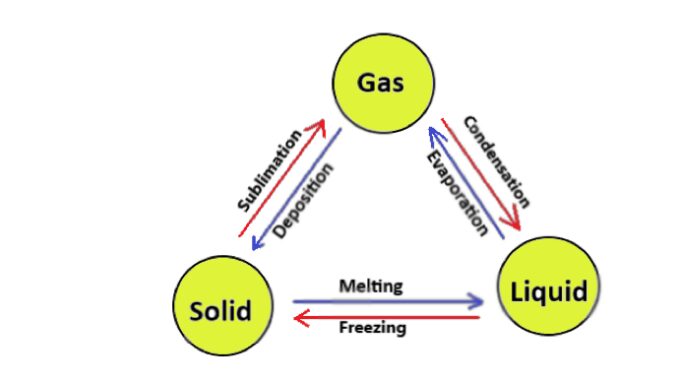
Introduction
Solid, liquid and gas are the three states of matter. Now a simple question arises in our mind what is a matter? Therefore, matter is simply defined as having mass and volume and occupying space. It also shows gravitational force and obstructing the use of force. In everyday life, we see many different things around us. Such as a table, book, chair, stone, milk, water, etc. These are matters. All matter is made by atoms, which are inseparable or cannot be broken.
Properties of Matter
- Matter has mass
- It has volume
- Most of the matter consists of atoms
- Matter shows the power of gravity
- The matter obstructs the application of force
Matters are further divided into three types based on the above properties. These three major states of matter are solid, liquid, and gas.
Basic definition and differences of solid, liquid, and gas three states of matter
Solid
Solid matter is one of the three main states of matter. A matter that has a certain shape and volume, whose shape and volume do not change under normal conditions, in which the molecules of the matter are in very close proximity and that cannot move from one place to another, is called a solid matter.
Examples of solid
Iron, wood, ice, brick, stone, coal, sand, etc. are solid matter.
Properties of solid matter
1. Shape: Solid has a certain shape. The shape does not change under normal conditions.
2. Volume: It has volume. Its volume does not change under normal conditions.
3. Nature of molecules: In solid matter, the molecules are very closely packed.
4. Melting and boiling point: Solid matter has a melting and boiling point above room temperature.
5. Flow: Solid matter cannot move from one place to another place.
6. Density: These are mostly high densities.
7. Structure of molecules: The molecules in the solid matter are closely proximate, so solids are hard.
8. Pressure: Solids are influenced by high pressure.
9. Kinetic energy: In solids matter, the particles have very little kinetic energy at room temperature.
Liquid
A matter that has a certain mass and volume, but has no shape and the matter whose molecules are constantly vibrating, is called a liquid matter. Most of the liquid matter in the world is non-metals. But mercury is the only metal that remains in a liquid state.
Examples of liquid
Water, milk, petrol, vegetable oil, blood, kerosene, mercury, ethanol, mineral oil, etc. are some examples of liquid matter.
Properties of liquid matter
1. Mass and volume: Liquid matter has a certain mass and volume.
2. Shape: It has no shape. It takes the shape of the container, where it is kept.
3. Nature of molecules: The molecules of the liquid matter are constantly vibrating.
4. Flow: The molecules of liquid matter can flow easily.
5. Density: Liquid matter has high densities, but less than solid matter.
6. Diffuse: Liquid matter molecules can scatter faster than solid matter.
7. Nature of force: Liquids have a less intermolecular force of attraction.
8. Kinetic energy: The molecules of a liquid matter have high kinetic energy.
9. Packing of molecules: In the liquid states, the molecules are loosely packed.
Gas
A matter that has a certain mass but no shape and volume is called a gaseous matter. It is a physical state of matter because by increasing the pressure and lowering the temperature, the matter can be turned into a liquid and subsequently it can be converted from liquid to solid in the same way.
Example of gas
Oxygen, carbon dioxide, nitrogen, hydrogen, etc. are some examples of gases matter.
Properties of gas matter
1. Mass: Gas has a certain mass.
2. Shape and volume: It has no shape and volume.
3. Nature of diffuse: The molecules of gases diffuse rapidly.
4. Melting and boiling point: The melting and boiling points of gas matter are below room temperature.
5. Density: The densities of gas matter are very low.
6. Kinetic energy: The particles of gas matter have very high kinetic energy.
7. Nature of molecules: The gas molecules are not very proximate so they are highly fluid in nature.
8. Nature of force: The force of attraction is very weak between the particles of gasses and matter (1).
Differences between solid, liquid, and gas
Based on the above information there are some differences between solid, liquid, and gas.
Properties |
Solid |
Liquid |
Gas |
Shape |
The shape of the solid does not change under normal room temperature and pressure. |
It has no shape. It takes the shape of the container, where it is kept.
|
There is no shape of gas and are therefore shapeless. |
Volume |
It has volume. Solid matter volume does not change under normal conditions.
|
The liquid matter has a certain volume. | Gases matter has no volume. |
Density |
The solids have relatively high density than that of liquid and gas. | Whereas liquids have a density higher than gas but lesser than solid matter.
|
The gas matter has the lowest density than that of solid and liquid. |
Melting & boiling point |
A solid matter has a melting and boiling point above room temperature.
|
The melting point of a liquid matter is below room temperature, and the boiling point is above room temperature. | The melting and boiling point of gases matter is below the room temperature.
|
Structure of molecules |
In solid matter, the molecules are very closely packed. So solids are hard.
|
In liquid matter, the molecules are slightly close. So liquids are fluid.
|
The gas molecules are not very proximate so they are highly fluid in nature.
|
Kinetic energy |
In solid matter, the particles have very little kinetic energy at room temperature.
|
The molecules of a liquid matter have high kinetic energy.
|
The particles of gas matter have very high kinetic energy.
|
The nature of molecules diffuse |
Solid matter cannot diffuse from one place to another place.
|
Liquid matter molecules can scatter faster than solid matter.
|
The molecules of gases diffuse rapidly.
|
Nature of force |
It has a strong intermolecular force of attraction. | Liquids have a less intermolecular force of attraction.
|
The force of attraction is very weak between the particles of gasses matter.
|
Flow |
The solid matter cannot flow from one place to another place. | The molecules of liquid matter can flow easily.
|
Gases can flow in all directions. |
Written By: Manish Bharati
References
Science for 9th class chemistry by Lakhmir Singh and Manjit Kaur. S. Chand School Books.
