Know in one minute about molecularity
|
Introduction
The branch of chemistry which deals with the study of reaction rates and their mechanisms is called chemical kinetics. Chemical kinetics helps us to determine how a reaction took place. Ot it helps in determining the time taken by a particular reaction to occur or whether a reaction would be spontaneous i.e. would proceed naturally or not. Hence, to fulfill all these needs we observe certain properties of the reactions. Molecularity is one such property.
These properties can be useful in various ways as one can predict the spontaneity of the reaction, the result, and the time taken by the reaction to occur precisely. In a study, it is found that the predictions did i.e. the theoretical data and the practical values may have differed but are very close for a huge number of cases. In this article, we will be going to discuss reaction types and its mechanisms.
The molecularity of a reaction is the number of reacting species taking part in an elementary reaction that must collide simultaneously in order to complete a chemical reaction.
OR
For a reaction, molecularity can be defined as the sum of all stoichiometric coefficients at the reactant’s side.
For example: 2HI → H2 + I2
The molecularity for the above reaction is 2.
Depending upon the number of species involved there are three types:
- Unimolecular
- BImolecular
- Trimolecular
1. Unimolecular
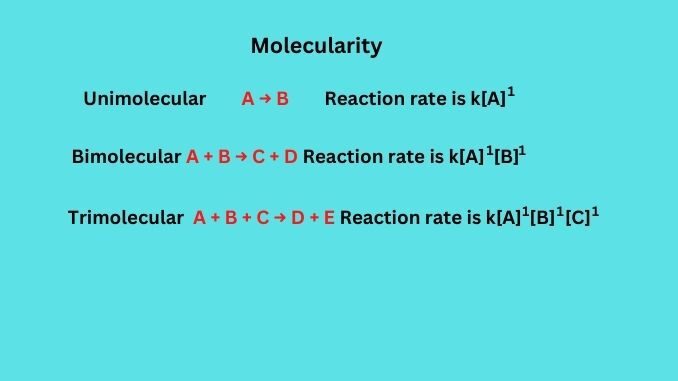
The reaction involving one reacting species is said to have molecularity of unity value i.e. one.
OR
For molecularity to be unimolecular the sum of reacting side stoichiometric coefficients must be one.
A → B
Rate of the reaction = k[A]1, where [A] is the concentration of the reactant.
Examples: Let us look at some examples to understand the concept better.
- NH4NO2 → N2 + 2H2O
This reaction is a decomposition reaction of ammonium nitrite into nitrogen gas and water. We can observe that the reaction has only one reactant and hence its molecularity is one and is unimolecular.
- O3 → O2 + O
Decomposition reactions are prominent examples of unimolecular reactions. The above reaction is also a decomposition reaction. In this reaction, ozone molecule is getting decomposed into oxygen gas and a nascent oxygen molecule.
- 2 N2O5 → 2 N2O4 + O2
- Some Radioactive decays are also an example of reactions falling under this type of molecularity.
Problems and their solutions on unimolecular
1. What is the molecularity of the radioactive active decay of U-235 an isotope of uranium?
Ans. Radioactive decay of U-235 is an example of a fission reaction, the U-235 atom splits stabler atoms. Hence this kind of reaction has 1 as its molecularity.
2. What must be the number of reactants for a reaction to have 1 as its molecularity?
Ans. There must be only one reactant.
Bimolecular
The reaction involving a simultaneous collision between two species is said to have a molecularity of two.
Or
When the sum of all the coefficients of the species at the reactant side becomes equal to two then the reaction exists with a molecularity of two.
A + B → C + D
Example: Some examples are stated below
- 2 HI → H2 + I2
- 2NO2 → N2O4
- CH3Br + OH– → CH3OH + Br –
- 2H2O2 → 2H2O + O2
- CH3COOH + C2H5OH → CH3COOC2H5 + H2O
Problems and their solutions on bimolecular
1. HCl + NaOH → NaCl + H2O
Comment on the molecularity of the reaction given above.
Ans. The above reaction has a molecularity of value two.
2. PhMgBr + PhCHO → PhCH(OH)Ph
The reaction above consists of a reagent, which is an important ingredient in forming many different types of compounds. Describe the reagent and the molecularity of the reaction.
Ans. It is Grignards reagent, and its general formula is RMgX. The molecularity of the reaction is 2.
Termolecular/ Trimolecular
The reactions containing collision between three reacting species are described to have molecularity of value three.
Or
In other words, a reaction is said to have Trimolecularity only if the algebraic sum of the coefficients of the reactants is three.
A + B + C → D + E
Generally, a reaction with Trimolecularity is of the form given above.
Examples
- 2SO2 + O2 → 2SO3
- CH4 + NH3 + O2 → HCN + 3H2O
- C2H4 + 2NO2 → 2NO + 2CO2 + 2H2O
- H2 + O2 + N2 → NH3 + H2O
- 2NO + O2 → 2NO2
In each of these reactions, three molecules or atoms collide to form the products. It is important to note that the molecularity of a reaction can only be determined experimentally by analyzing the reaction rate and identifying the rate-determining step. The number of molecules or atoms involved in the rate-determining step determines the molecularity of the reaction.
Problems and their solutions on Termolecular/ Trimolecular
1. In a complex reaction containing multiple steps how do we calculate molecularity?
Ans. In order to calculate the molecularity of a complex reaction :
- Firstly write the balanced chemical equation for the reaction.
- Identify the rate-determining step of the reaction. This is the step that limits the overall rate of reaction.
- Calculate the number of molecules involved in the rate-determining step.
- This is the molecularity of the reaction.
2. Can a reaction possess a molecularity greater than 3?
Ans. Yes, a reaction can have a molecularity greater than 3, but practically this condition is very rare. Reactions having more reactants are comparatively unstable in nature as collisions in the reaction increase resulting in the instability of the reaction.
Written By: Bharat Awasthi
