Know in one minute about Basic amino acids
|
Introduction
Basic amino acids are positively charged, groups with an amino group (NH2+) in their side chain. They are hydrophilic in nature that is side chain has an affinity to water.
The arrangements of atoms in a molecule help determine its polarity. A molecule is said to be polar if it has a positive and negative charge. Basic amino acids are polar and positively charged.
Amino acids are joined to each other by peptide bonds to form proteins and peptides. The sequence of amino acids forms the backbone of proteins. There are approximately 300 amino acids present in animal, plant, and microbial systems, but only 20 amino acids are involved in the formation of protein. All the 20 amino acids found in proteins have a carboxyl group (-COOH) and an amino acid group (-NH2) bound to the same carbon atom called the α-carbon.
Classification based on the chemical nature
1. Neutral amino acids
They are neutral in solution with monoamino and monocarboxylic acids (i.e. they have one carboxylic group and one amino group)
Examples are Alanine, glycine, Valine, Leucine, Isoleucine, Serine, Threonine, cysteine, Methionine, Proline, Phenylalanine, Tyrosine, Tryptophan, Asparagine, and Glutamine.
2. Acidic amino acids
These are acidic in solution and are monoamino- dicarboxylic acids (i.e. they have one amino and two carboxyl groups). Examples are Glutamate, Aspartate.
3. Basic amino acids
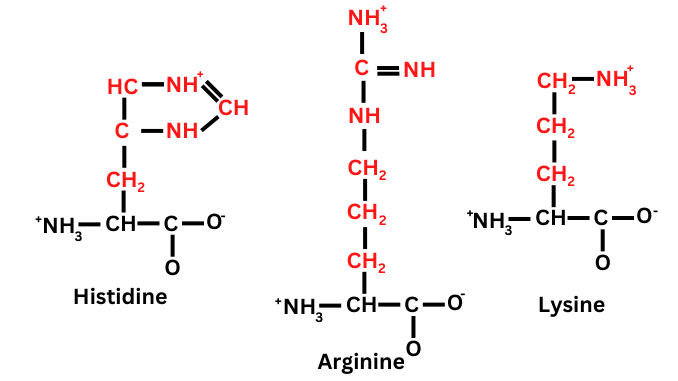
These are basic in solution and diamino-monocarboxylic acid (i.e. they have one group of carboxyl and two groups of amino). Examples are Histidine, Arginine, Lysine.
Examples of Basic amino acids
1. Arginine
- Arginine also has a relatively long side chain group. A terminal portion is a guanidinium group that is positively charged at a neutral pH.
- It is synthesized from Glutamate which is an acidic amino acid. Arginine has the three-word abbreviation “Arg” and the one-word abbreviation “R”.
- Arginine is a semi-essential amino acid. It can be synthesized in the body but not in sufficient quantities. It is essential for growth.
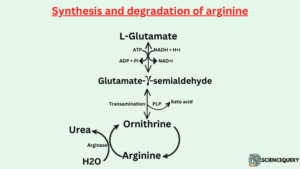
Degradation of Arginine
- Arginine is cleaved by arginase to liberate urea and produce ornithine.
- Ornithine undergoes transamination of the δ-amino group to form glutamate γ-semialdehyde which is converted to glutamate.
Synthesis of Arginine
- Arginine is synthesized from glutamate.
- Glutamate is reduced to glutamate-γ-semialdehyde.
- Which is then transaminated to yield ornithine, an intermediate of the urea cycle. The reactions of the urea cycle convert ornithine to Arginine.
2. Histidine
- Histidine contains the imidazole ring which is an aromatic ring. Histidine can exist in its protonated or deprotonated state at neutral pH.
- It is synthesized from glutamate. Three words abbreviation of Histidine is “His” and one letter abbreviation is “H”.
- Histidine is a basic and semi-essential amino acid which means it is synthesized partially in the human body.
- They are responsible for the maximum buffering action.
- Its pKa value is 6.1.
Note: pKa value describes the acidity of an amino acid or other molecules. A low pKa value indicates strong acid and a high pKa value for the alkalinity of molecules.
3. Lysine
- Lysine has a relatively long side chain group that is contained by the primary amino group.
- It is synthesized from acetoacetyl CoA. “Lys” is a three-letter abbreviation and “K” is a one-letter abbreviation of lysine.
- Lysine metabolized to Acetyl CoA or acetoacetate. Acetoacetate is a ketone body.
- It is a nutritionally essential amino acid (That is cannot be synthesized by the body).
- Lysine is a Ketogenic amino acid (Amino acids can be converted into ketone bodies through ketogenesis).
- Does not participate in transamination.
- Lysine residues present in protein as hydroxylysine, methyllysine, or acetyllysine. Ε-Amino group of lysine.
- They formed salt bridges which are essential for the maintenance of the structural conformation of the protein.
Metabolic disorders of basic amino acids
Metabolic disorder of Arginine
Hyperargininemia is an inborn error in arginine metabolism due to a defect in the enzyme arginase.
Metabolic disorder of Histidine
Histidinemia
Histidinemia is characterized by elevated blood and urine histidine. The defective Enzyme is histidase, resulting in the conversion of histidine to urocanate.
Urocanic aciduria
It is characterized by elevated excretion of urocanate, which results from a defective urocanase enzyme.
Metabolic disorder of Lysine
Periodic hyperlysinemia
It is characterized by hyperammonemia and elevated plasma lysine. Elevated liver lysine levels competitively inhibit Liver arginase, causing hyperammonemia. It shows the clinical symptoms of ammonia Intoxication.
Persistent hyperlysinemia
Persistent hyperlysinemia is believed to be inherited as an autosomal recessive trait.
How are basic amino acids different from normal amino acids
1. Structural difference
- Have a greater number of amino groups (-NH2), other normal amino acids are monoamine acids.
- Positively charged.
- Are positively charged and others are uncharged amino acids.
2. Chemical nature of amino acids
They accept the proton in the solution and act as basic in nature. Normal amino acids
3. Buffering action of amino acids
Histidine serves as the best buffer at physiological pH because the side chain of Histidine has a pKa of 6.0.
On the other hand, normal amino acid’s pKa value is too far away from pH 7 to be an effective physiological buffer.
Maximum buffer capacity occurs at pH equal to the pKa.
4. Charged polar side chain
Five of the twenty amino acids have side chains with full charges of the normal physiological pH. This makes them highly hydrophilic. Therefore all basic and acidic amino acids are hydrophilic.
Structure of basic amino acid
Basic amino acid basically contains a carboxyl group (-COOH-), hydrogen, an amino group (-NH2), and a side chain or ‘R’ group.
Structure of Lysine
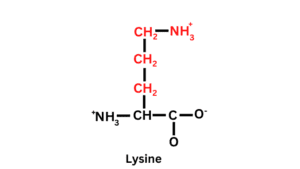
Lysine has a long side chain. Lysine contains one carboxyl group (-COOH-), one Hydrogen, and one amino group (-NH2), and in the side chain, (CH2)4 -NH2 is attached.
Structure of Arginine
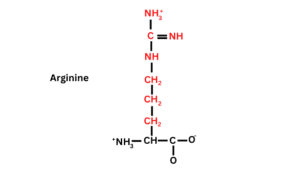
Arginine is the most basic amino acid. It is made with one carboxyl group (-COOH-), one Hydrogen, one amino group (-NH2), and a guanidine unit attached in their side chain.
Structure of Histidine
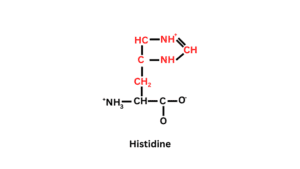
Histidine is made with one carboxyl group (-COOH-), one Hydrogen, one amino group (-NH2), and an imidazole ring attached in their side chain. It is an aromatic ring.
Difference between Basic amino acids and Acidic amino acids
Features |
Basic amino acids |
Acidic amino acids |
Definition |
These are basic in solution and are diamino–monocarboxylic acids. | Are acidic in solution and are monoamine-dicarboxylic acids. |
Examples |
Glutamate (Glu), Aspartate (Asp) | Arginine (Arg), Histidine (His), Lysine (Lys) |
Composition |
Contain more than the number of ammonia (-NH4+) groups or positively charged groups in comparison to negatively charged groups. | Contain more than the number of carboxylic (-COOH–) groups or negatively charged groups in comparison to positively charged groups. |
pKa value |
Have a high pKa value. | Low pKa value. |
Synthesis |
Basic amino acids are formed from acidic amino acids. Arginine and histidine are synthesized from Glutamate which is the acidic amino acid. | Acidic amino acids formed from TCA cycle intermediates. Aspartate is synthesized from oxaloacetate and Glutamate is synthesized from alpha-ketoglutarate. |
Status |
They are semi-essential except lysine which is a nutritionally essential amino acid. Semi-essential means they are partially synthesized in the human body. | Are nonessential amino acids. These types of amino acids can be synthesized by the body, so we don’t need to take them in our diet. |
Glucogenic or Ketogenic character |
Have Ketogenic properties. They produce ketone bodies through ketogenesis. Ex. Lysine | Glucogenic properties. They produce glucose through gluconeogenesis. Ex. Aspartate, Glutamate. |
Charge |
More number of positively charged groups in comparison to positive charge groups. That’s why these are called hydrophilic positively charged amino acids. | Are hydrophilic and negatively charged because they have more than no. of negatively charged groups compared to positively charged groups. |
Proton acceptor or donor |
Accept protons (H+) and act as basic | while carboxylic groups of acidic amino acids donate protons (H+) and act as acids. |
Uses of basic amino acids
Arginine uses
- Arginine takes part in the formation of urea.
- It is involved in the synthesis of creatine, An important constituent of muscle.
- In the formation of creatine, the guanidinium group of Arginine is transferred to glycine.
- Nitric oxide is synthesized from arginine Nitric oxide is a biological messenger in a variety of physiological responses including Vasodilation, neurotransmission, and the ability to Kill tumor cells and parasites.
Histidine uses
The metabolism of histidine is important for the generation of one carbon unit, namely forming an imino group.
- Purine synthesis
Histidine during its degradation produces Formimino glutamate (FIGLU), which reacts with THF forming N5-forming THF which on releasing ammonia generates N5, N10-methenyl THF. This N5, N10 methenyl THF is used in purine Synthesis.
- Production of vasodilator by decarboxylation
Vasodilator produced by decarboxylation of Histidine and Lysine.
- Synthesis of Histamine
Histamine is produced by decarboxylation of histidine. It is a vasodilator and lowers blood pressure.
It is involved in asthma and allergic reactions.
Histamine regulates HCl secretion by the gastric mucosa.
- Synthesis of carnosine and anserine
Histidine synthesized, biologically important peptides like carnosine and anserine present in muscle, ergothioneine present in erythrocytes, liver and brain are synthesized from it.
Lysine uses
- Hydroxyl lysine is important for the covalent crosslinking of Collagen.
- Decarboxylation of Lysine forms cadaverine.
- Lysine is involved in the synthesis of carnitine, Carnitine plays an important role in the fatty acid oxidation.
Q&A
1. How many amino acids are there?
Approximately 300 are found in animal, plant, and microbial systems but only 20 are involved in Protein synthesis.
2. What essential amino acids?
They can not be synthesized by the body. It is essential to take in our diet.
3. How many essential amino acids are there?
11 examples are Phenylalanine, Methionine, Valine, Histidine, Threonine Arginine, Tryptophan, Lysine, Isoleucine, and Leucine are the essential amino acids. In there Arginine and Histidine are semi-essential.
4. How many amino acids in a protein?
20 present in a protein.
5. How many amino acids make up a protein?
20 amino acids that are responsible for protein synthesis.
6. How many non-essential amino acids are there?
Nonessential amino acids can be synthesized in the human body and are not required in the diet. Ex. Glycine, Alanine, Proline, Tyrosine, Serine, Cysteine, Glutamic acid, Aspartic acid, Glutamine, and Asparagine.
7. How many amino acids per day?
There are 9 essential amino acids that must be taken in the diet per day.
Written By: Richa Pachori
References
1. Essentials of Biochemistry, Pankaja Naik
2. Biochemistry 4th edition, U. Satyanarayana & U. Chakrapani
