Know in one minute about Peptide bond formation
|
Introduction
What is a peptide Bond?
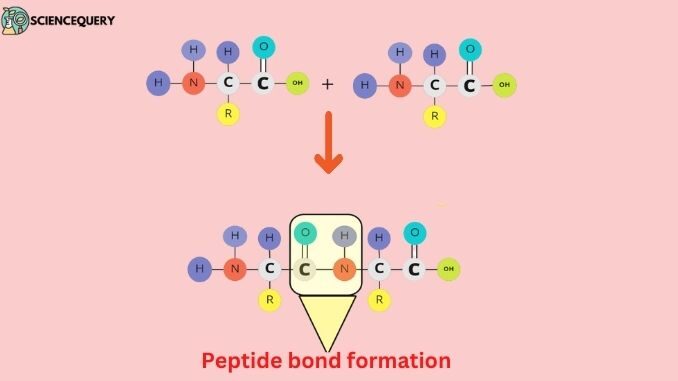
A peptide bond is a bond or linkage (—CO–NH—) between two amino acid units that are joined through a dehydration reaction. During this reaction there is the formation of a bond between two amino acids by the elimination of water molecules, this bond is known as a peptide bond or an amide bond. Peptide bond formation occurs only in long-chain proteins.
Features of Peptide Bonds
1. Partial Double Bond Character
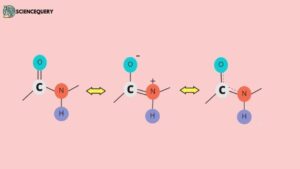
- Peptide bonds are represented by a single bond between α-carboxyl and α-nitrogen atoms. But interestingly, this bond exhibits a partial double-bond character.
- Due to this, the bond that connects carbonyl carbon and nitrogen has no freedom of rotation about its axis.
- Because of the partial double bond character, the O, C, N, and H atoms of a peptide bond are coplanar (that is they are in the same plane).
- The resultant semi-rigidity due to the partial double bond character governs the folding of peptides and proteins while forming higher-order molecular structures.
2. Peptide bonds are strong in nature
Even high salt concentrations or heat cannot break them. Only subjecting them to high temperatures in presence of strong acids or bases over a prolonged period of time or exposing them to certain enzymes such as “Proteases” can denature peptide bonds.
3. Peptide bonds are stiff and planar
This characteristic of peptide linkages stabilizes the structure of proteins.
4. The polarity of the peptide bond
Like all amide linkages, the –C =O and –NH groups of the peptide bond are non-Polar in nature because of which they neither accept nor release protons over the pH range of 2–12.
Thus, the charged groups present in polypeptides consist solely of the N-terminal (α-amino) group, the C-terminal (α-carboxyl) group, and any ionized groups present in the side chains of the constituent amino acids.
Importance of peptide bonds
- Peptide bonds are the foundational backbone of proteins, Therefore, they are of utmost significance in biochemistry.
- The formation of the peptide bond is the most puzzling aspect of the emergence of life; that is, to find how amino acids are activated and peptides are created at the origin of life.
- Our human body stays intact only because of peptide bonds; even the entire living structure is established on protein, all thanks to peptide bonds, which act as a chain to join the amino acid sequence, which subsequently folds to form proteins due to numerous internal and external pressures.
Mechanism of peptide bond formation
- The process of dehydration synthesis is the mechanism of peptide bond formation.
- During peptide bond formation, the carboxyl group of one amino acid migrates toward the amino group of another amino acid.
- Subsequently, the carboxyl group (COOH) of the first amino acid loses one hydrogen and one oxygen atom. In contrast, the amino group (NH2) of the other amino acid loses one hydrogen.
- During peptide bond formation, a water molecule (H2O) is released, and a C-N amide bond is created between the two amino acids.
Main component released during peptide bond formation
Water molecule is the key component released during peptide bond formation as a result of the de-hydrolysis reaction.
Systematic events in peptide bond formation
Peptide bond formation involves several systematic events, which are as follows:
Step 1. Carboxyl group activation
The first step in peptide bond formation is the activation of the carboxyl group of the amino acid that will act as the donor. This involves the conversion of the carboxyl group into a more reactive form, such as an ester or an acid anhydride, which can react with the amino group of the acceptor amino acid.
Step 2. Nucleophilic attack by the amino group
The activated carboxyl group of the donor amino acid then reacts with the amino group of the acceptor amino acid, leading to the formation of an intermediate molecule called an acyl-enzyme intermediate. This intermediate is formed by nucleophilic attack by the amino group on the carbonyl carbon of the carboxyl group.
Step 3. Elimination of H2O
In the next step, a molecule of water is eliminated from the acyl-enzyme intermediate. Therefore, resulting in the formation of a peptide bond between the two amino acids. This process is also called a dehydration synthesis reaction.
Step 4. Peptide Bond Formation
The resulting molecule is a dipeptide, which contains a peptide bond between the carboxyl group of one amino acid and the amino group of the other amino acid. This process can be repeated to form longer peptide chains.
Degradation of Peptide Bond
- The degradation of peptide bonds is an important biological phenomenon. It occurs within a cell and is catalyzed by enzymes called proteases.
- There are different ways through which proteases break the peptide bond. Depends upon the types of protease and specific amino acid sequences present in the protein.
- Hydrolysis is one of the ways through which proteases break the peptide bond by the addition of water molecules to the peptide bond.
- The nucleophilic attack is another prevalent way of cleaving peptide bonds. In which reactive amino acid residue in protease attacks the peptide bond resulting in its fragmentation and release of amino acids.
- Some examples of proteases are serine proteases, cysteine proteases, and aspartic proteases. These proteases carry different amino acid residues at their active sites. Which allows them to cleave specific peptide bonds in proteins.
Q&A
1. State the proper sequence of events during the peptide bond formation?
The sequence of events in peptide bond formation involves the following steps:
Activation of the carboxyl group: The carboxyl group (-COOH) of the first amino acid is activated by conversion to a more reactive intermediate. These are called aminoacyl groups (-CO-NH-). This reaction is, therefore, catalyzed by the ribosome and requires the hydrolysis of ATP to provide energy.
Formation of a peptide bond: The activated carboxyl group of the first amino acid reacts with the amino group (-NH2) of the second amino acid, forming a peptide bond (-CO-NH-) and releasing a molecule of water (H2O).
2. Which of the following components emerges when a peptide bond is formed?
a) NH3 b) H2O c) CH4 d) CO2
A water molecule (H2O) is released as a result of the peptide bond formation due to the condensation of two amino acids. In which the carboxyl group (-COOH) of one amino acid interacts with the amino group (-NH2) of another amino acid in the sequence.
Written By: Deva Singh
