Introduction
Oxidative phosphorylation is the production of ATP by chemiosmosis. It is the terminal oxidation of aerobic respiration. During the respiration process, food is oxidized in the cell, and energy is produced. This energy regulates the physiological activities of the organism.
When food is oxidized in the presence of oxygen, it is called aerobic respiration. This process takes place in the cells of the organisms hence it is known as cellular respiration. The oxidation process of food is completed through different stages and finally, energy is generated.
Cellular respiration or aerobic respiration is completed in four steps. In the first three steps, ATP and the other two energy compounds namely NADH₂ and FADH₂ are produced. In the last stage, ATP is generated from these two compounds.
The process of ATP molecule formation takes place in this last stage of respiration through the electron transport chain and chemiosmosis. It is a combination process of electron transport chain and chemiosmosis. That is, it is the last stage of cellular respiration where ATP molecules are formed. This stage is discussed in detail below (4) & (7).
Oxidative phosphorylation
Glucose molecules are fully oxidized in the Krebs cycle process. But energy (ATP) is not available until NADH₂ and FADH₂ are fully oxidized. NADH₂ and FADH₂ cannot react directly with oxygen.
This reaction occurs step by step and ATP is generated. The synthesis of ATP by the oxidation of these hydrogen receptors (NADH₂ and FADH₂) with the help of energy is oxidative phosphorylation.
NADH₂ and FADH₂ are oxidized by two processes. These are electron transport chains and chemiosmosis. Two compounds NADH₂ and FADH₂ donate their electrons to the electron transport chain.
Thus the electrons move from one carrier to another through the electron transport chain. An electrochemical gradient is formed where the electrons move. The electrochemical gradient is used to apply energy in this process.
On the other hand, chemiosmosis uses this gradient to form ATP. In this way, adenosine triphosphate is produced in two processes of oxidative phosphorylation. This process produces 28 molecules of ATP which is much higher than the ATP molecules produced in the glycolysis process (4) & (6).
Definition
Oxidative phosphorylation is an aerobic process where the oxidation of food occurs in the presence of oxygen. And high potency ATP is produced by combining inorganic phosphate compounds with ADP (5).
Who discovered oxidative phosphorylation?
Arthur Harden began the field of oxidative phosphorylation in 1906 while describing the role of phosphate in cellular fermentation. Then in early 1940, when describing the oxidation of glucose and the synthesis of ATP, Herman Kalckar gave some information about this process.
In 1941, biochemist Fritz Albert Lipmann described this stage in describing the role of ATP in energy transfer.
Later various scientists discussed the process. But the term oxidative phosphorylation was discovered by Volodymyr Belitser in 1939. Later, in 1961, the chemiosmosis theory of Peter D. Mitchell explained in detail that ATP is produced by oxidative phosphorylation in the mitochondria (1) & (3).
Location
It is a process in which ATP is synthesized by the electron transfer of NADH₂ and FADH₂ to O₂. This process occurs in all eukaryotic and prokaryotic cells. In eukaryotic cells, it is located in the mitochondria.
The inner membrane of the mitochondria is the ideal site for this process to take place. It occurs in the plasma membrane of the prokaryotic cells (4) & (5).
Relationship between chemiosmosis and oxidative phosphorylation
Oxidative phosphorylation has two components. Chemiosmosis is one of them. Chemiosmosis is the process of diffusion of ions across a semipermeable membrane under an electrochemical gradient.
It is a mechanism by which ATP is produced in cellular respiration. In this process, the energy is used to pump hydrogen ions across the membrane. as a result, an electrochemical gradient is formed. The process of ATP (Adenosine triphosphate) formation by chemiosmosis in mitochondria is known as oxidative phosphorylation. So these two processes are related to each other (1) & (2).
Features
1. This is a metabolic pathway.
2. Metabolic processes require a lot of energy. The main source of this energy is ATP. Oxidative phosphorylation is a major method for producing ATP.
3. The presence of oxygen in this process is extremely important. So this process is a stage of aerobic respiration.
4. ATP synthase is an enzyme that participates in this process of cellular respiration.
5. The process is completed in mitochondria.
6. Main purpose of this process is to generate ATP. A phosphate group (Pi) is added to ADP and adenosine triphosphate is generated.
7. Here the energy released from the electron transport chain is used to generate energy (ATP).
8. It is an indirect phosphorylation process.
9. Here the oxidation of NADH⁺ and FADH⁺ occurs.
10. It consists of two components, such as electron transport chain and chemiosmosis (6) & (7).
Why is it called oxidative phosphorylation?
Oxidative phosphorylation is a very important metabolic process. It occurs mainly in mitochondria. Here ATP is formed by the addition of ADP and inorganic phosphate (Pi).
Phosphorylation refers to the transfer of a phosphate group from one compound to another. Transformation of these phosphate groups occurs in the presence of oxygen. Therefore this process is called oxidative phosphorylation (4).
Step
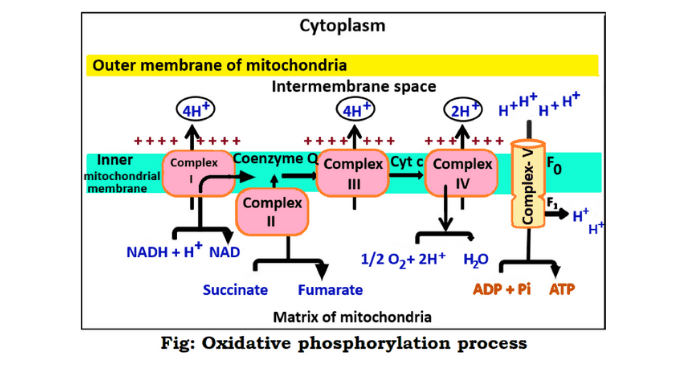
This process is the terminal process of aerobic respiration of eukaryotic cells. The process is completed through a few steps. Such as-
1. Oxidation of NADH₂ and FADH₂
The first step of this process is the oxidation of NADH₂ and FADH₂. Here the electrons are released from NADH₂ and FADH₂. These electrons move from one carrier to another through an electron transport chain. After the transformation of the electrons, these components oxidized to NAD⁺ and FAD⁺. NAD⁺ and FAD⁺ are used in another process of respiration.
2. Pumping and electron transformation of protons
Electrons are generated in the first step and move from a higher density region to a lower density region. As a result, energy is emitted from these electrons. It takes a lot of energy to transfer electrons from the matrix to the intermembrane space. The energy emitted from the electrons participates in this transfer process.
3. Breakdown of oxygen in the formation of water
Electrons of NADH₂ and FADH₂ are transferred to the electron transport chain. These electrons move from higher concentration to lower concentration and eventually attach to the oxygen. After transfer to the oxygen molecule, electrons break down into two parts. The breakdown of electrons takes up hydrogen ions (H⁺) to form water.
4. The synthesis of ATP
It is the final step of oxidative phosphorylation. When H⁺ ions flow, they pass through ATP synthase present in the mitochondria of the cell. ATP synthase is an enzyme. It is the primary enzyme in generating ATP in the oxidative phosphorylation process. It is known as complex-V, PTAS (Proton Translocating ATP synthase).
This enzyme causes the movement of protons or hydrogen ions. The protons of hydrogen ions generate energy only when their concentration is higher in the intermembrane space than in the matrix and ADP is sufficiently available.
These hydrogen ions re-enter the matrix through the ATP synthase enzyme to produce ATP and complete ATP synthesis. The enzyme ATP synthase combines inorganic phosphate with ADP to produce ATP. Thus ATP is generated at the last stage of cellular respiration (6) & (3).
Products
The product that is produced at the end of this process is ATP (adenosine triphosphate). About 28 molecules of ATP are produced in this process. Besides, water is formed when oxygen receives the electrons from the electron transport chain (Complex- IV) and combines with hydrogen ions inside the mitochondria of the cell (5).
How electron transport chain and oxidative phosphorylation related?
It occurs when electron transport chains and chemiosmosis combine. Here phosphorylation of ADP occurs and ATP is generated. The power of the proton gradient established by the electron transport chain is used in this process.
The formation of a proton gradient depends on the electron transport chain. If the transfer of electrons to the electron transport chain is stopped for any reason, oxidative phosphorylation will no longer occur. This is because ATP will not be generated if the ATP synthase (enzyme) is not driven by proton gradients (5) & (2).
Importance
1. It is an essential part of carbohydrates metabolism.
2. The main source of energy in the aerobic respiration or cellular respiration process is ATP, which is generated in this process.
3. The process produces hydrogen peroxide and superoxide. These are reactive oxygen species.
4. Electrons are exchanged between molecules during this process. As a result, an electrochemical gradient is produced which allows for the production of ATP.
5. NADH₂ and FADH₂ provide their electrons to the electron transport chain. The oxidation of NADH₂ and FADH₂ occurs as a result of providing electrons and these are converted into NAD⁺ and FAD⁺. They are used in other processes of cellular respiration and metabolism (1) & (3).
Q&A
1. What is oxidative phosphorylation?
The process by which food is oxidized in the presence of oxygen and high-energy molecules ATP is produced by the combination of Pi and ADP in the mitochondria of eukaryotic cells is known as oxidative phosphorylation.
2. Where does oxidative phosphorylation occur?
The process occurs in both eukaryotic and prokaryotic cells. In eukaryotic cells, the inner membrane of the mitochondria and the cytoplasmic membrane of prokaryotic cells are the ideal place for oxidative phosphorylation.
3. Where does oxidative phosphorylation takes place?
Eukaryotic cells have many cell organelles. Mitochondria is one of them. The process takes place in the inner mitochondrial membrane. In prokaryotes, it completes in the plasma membrane because this cell has no cell organelles.
4. Which of the following is the best definition of oxidative phosphorylation?
A proton gradient enables hydrogen ions to flow back into cells through transmembrane protein channels, expressing the energy that is used to produce ATP.
5. How much ATP is produced in oxidative phosphorylation?
28 molecules of ATP are produced in this process which is much higher than the ATP molecules produced in the glycolysis process.
References
1. B. Powar and G. R. Chatwal. Biochemistry, B. SC (general & honours course) and M. Sc. Himalaya publishing house, Chapter: Metabolism of carbohydrates. Page no- 539 to 564.
2. B Agarwal and V. K. Agarwal. Unified Botany, B.Sc. second Year. Shiva Lal Agarwal & Company Publications, Indore. Chapter: Respiration. Page no- 159 to 160.
3. Ajoy Paul. Zoology Honours, volume- 2, Books & Allied (P) Ltd. Chapter: Oxidative phosphorylation. Page no- 341 to 347.
