Introduction
Proteins are complex compounds that form nitrogen. However, before they can be absorbed by our intestines, they must be broken down into smaller units. This is because proteins have a complex structure that makes them difficult for our intestines to absorb. In this article, we will discuss the process of protein digestion in simple steps.
What is Protein digestion?
Protein digestion is a process involving the hydrolysis of large protein molecules into smaller peptides and amino acids.
Which can be easily absorbed by the gastrointestinal tract for utilization by the Organism.
The protein digestion and absorption are obtained from two sources, dietary and endogenous.
The intake of dietary protein is in the range of 50–100 g/day.
About 30–100 g/day of endogenous protein is derived from the digestive enzymes and worn-out cells of the digestive tract.
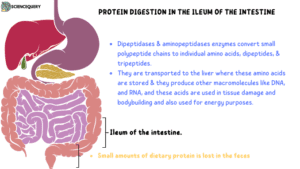
The digestion and absorption of proteins are very efficient in healthy humans, hence very little protein (about 5–10 g/day) is lost through feces.
Dietary proteins are denatured on cooking and therefore, easily digested.
Class of enzymes that are involved in protein digestion
Hydrolases
These enzymes cleaved peptide bonds, hence they are known as peptidases. They are divided into two groups.
1. Endopeptidases (proteases)
This enzyme cleaved internal peptide bonds and released peptide fragments. Examples are pepsin, trypsin
2. Exopeptidases
This enzyme acts on peptide bonds of terminal amino acids. Carboxypeptidases and aminopeptidases are subgroups of exopeptidases. Former acts on C terminal amino acid and aminopeptidases act on N terminal amino acid.
The proteolytic enzymes are responsible for Protein digestion. These are produced by the stomach, the pancreas, and the small intestine.
Where does protein digestion begin?
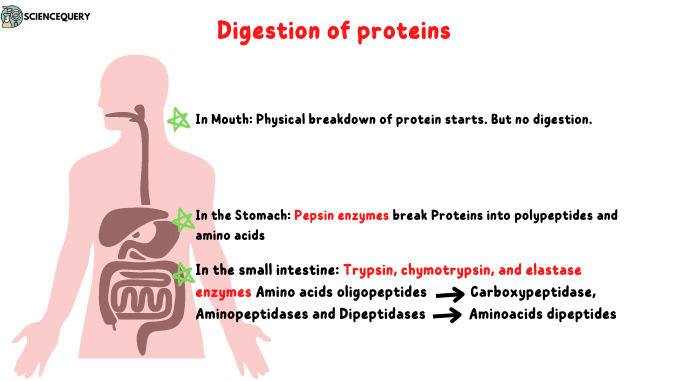
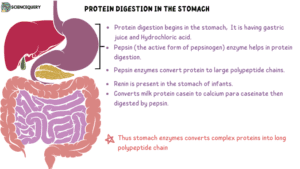
Digestion of dietary proteins begins in the stomach.
The cells lining the stomach produce and release gastric juice that contains hydrochloric acid and a proenzyme called pepsinogen.
Decreasing the level of pH of the stomach affects protein digestion because pepsin enzymes activate and work at low pH.
It is an acid-stable endopeptidase. It cleaves peptide bonds formed by amino groups.
Enzymes present in the stomach
1. Gastric acid
Hydrochloric acid lowers the pH to about 2-3. This acts as an immune defense against pathogens such as bacteria. In addition, it denatures the structure of proteins and makes them more susceptible to enzymatic cleavage. It also activates pepsin.
2. Pepsin
Pepsin enzyme is produced by serous cells of the stomach.
Pepsinogen is an inactive form and must be activated to pepsin by hydrochloric acid or by other active pepsin enzymes at a very low pH (2.0).
Once activated pepsin cleaves Protein into polypeptides. This enzyme degrades the protein molecules into proteoses and peptones.
Removal of a fragment of polypeptide Chain (44 amino acids in the case of pig enzyme) makes the inactive enzyme active after attaining a proper conformation.
The active site of the enzyme contains 2 carboxyl groups, which are maintained At low pH. Pepsin A is the most predominant gastric protease which preferentially cleaves peptide bonds formed by amino groups of phenylalanine Or tyrosine or leucine.
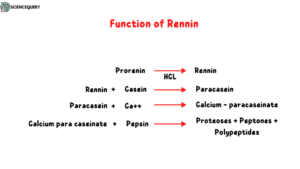
3. Rennin
This enzyme is found in the stomachs of infants and children. It converts milk protein casein to calcium para caseinate which can be effectively digested by pepsin. This enzyme is absent in adults.
Protein digestion in the Intestines
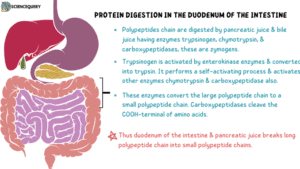
Once the polypeptides reach the intestine, they begin further breakdown by the activity of pancreatic enzymes such as trypsin, chymotrypsin, elastase, and carboxypeptidase.
These enzymes are secreted by the pancreas in their zymogen form as a result of two polypeptide hormones cholecystokinin and secretin.
Each of these enzymes has a certain specificity. That is, they cleave peptide bonds adjacent to specific amino acid R-groups.
An enzyme called enteropeptidase is responsible for converting trypsinogen to trypsin.
Trypsin then activates all the other pancreatic zymogens. Enteropeptidase itself is present on the luminal surface of the intestinal mucosa.
Clinical aspects
Some people develop pancreatic insufficiency (i.e. chronic pancreatitis surgical resection, cystic fibrosis) that leads to a deficiency in pancreatic enzymes. This causes the inability of the person to digest protein and fats leading to steatorrhea (the presence of fat and undigested protein in stool) (1).
Pancreas
Pancreatic enzymes cleave polypeptides into smaller units called oligopeptides.
These are then further degraded by luminal surface proteases called aminopeptidases into individual amino acids and di-tripeptides.
Once inside, the di-tripeptides are further broken down into amino acids before being released into the portal circulation.
The luminal surface of intestinal epithelial cells contains aminopeptidases and Dipeptidases.
Aminopeptidase is a non-specific exopeptidase that repeatedly Cleaves N-terminal amino acids one by one to produce free amino acids and smaller peptides.
The peptidases act on different dipeptides to liberate amino Acids.
These amino acids and small peptides are absorbed into the enterocytes via membrane transporters.
Once in the liver, the amino acids are either degraded, used to make other biological molecules, or secreted into the circulation (amino acid pool)
Transport of amino acids
Levels of amino acids inside cells are much higher than in the extracellular space due to the presence of ATP-driven membrane transporters. These are responsible for actively pumping amino acids into the cells through the hydrolysis of ATP.
Clinical aspects
Cystinuria is an autosomal recessive condition due to a mutation in the amino acid transporter of the proximal convoluted of the kidneys that’s normally responsible for the absorption of cysteine, lysine, arginine, and ornithine. This causes these amino acids to appear in the urine. In addition, the presence of cysteine in the tubules and duct can precipitate kidney stones (2).
Products of protein digestion
- Protein is a complex macromolecule. It consists of a larger polypeptide chain of amino acids.
- Amino acids join together with peptide bonds. Proteins contain 20 types of amino acids which are called standard amino acids.
- This enzyme is used in repairing tissue damage, and bodybuilding, as an energy source and to produce other essential proteins to fulfill body needs, the body digests proteins into free amino acids with the help of proteases enzymes.
- We easily understand what the products formed in protein digestion are with these reactions.
Products that formed in the stomach
Pepsin
In the stomach, HCl denatures the 3D structure of protein and activates the pepsinogen enzyme to pepsin. Pepsin cleaves the internal peptide bond of protein and converts protein to a large polypeptide. Pepsin enzymes formed proteases and peptones.
Peptic reaction
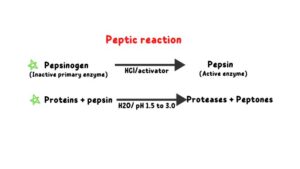
Rennin
In the presence of HCl prorennin converts to rennin. This enzyme reacts with casein milk and forms paracasein then paracasein forms calcium para caseinate reacts with calcium ions. This calcium para caseinate is favorable for pepsin activity. Pepsin enzymes digest this protein and form proteoses, peptones, and polypeptides.
Pancreatic enzymes
Chymotrypsin and trypsin are important pancreatic enzymes that convert long polypeptide chains to small polypeptide chains these enzymes are activated by the enterokinase enzyme
Trypsinogen → Enterokinase Trypsin
Chymotrypsin trypsin → Chymotrypsin
Protein → (Trypsin) Peptones + Polypeptides
Protein → (Chymotrypsin) Peptones + Polypeptides
The activity of carboxypeptidase, aminopeptidase, and Dipeptidases
Carboxypeptidases act on COOH-terminals of amino acids and aminopeptidases enzymes act on N-terminals of enzymes. All three enzymes convert small polypeptides to dipeptides, tripeptides, and free amino acids.
Importance and functions
- Protein digestion is crucial for the body to obtain the necessary amino acids that form the building blocks of proteins.
- Proteins are essential for various functions in the body, including bodybuilding, enzyme and hormone formation, and DNA and RNA synthesis.
- Moreover, proteins play a crucial role in repairing tissue damage and transporting molecules, such as oxygen and carbon dioxide, through protein channels.
- Without adequate protein digestion, the body cannot meet its protein requirements, which can result in various health issues.
- Therefore, protein digestion is critical for the proper functioning of the body and maintaining optimal health.
Functions
1. Source of energy
protein is a source of energy. Energy is generated from the breakdown of protein.
2. Enzymes synthesis
Enzymes are proteins. These are involved in biological processes and catalyze the reaction. Enzymes provide a site for substrate and convert to the product.
3. In hormones synthesis
Some proteins are involved in hormone synthesis. Hormones are released by the endocrine gland and released in the bloodstream. These initiate biochemical reactions and work as chemical messengers.
4. Structural and building materials
Protein is a structural material. Actin, myosin, collagen, elastin, and keratin are proteins that make tissues, strength, and structure.
5. Muscle contraction
Proteins are responsible for muscle contraction. Actin and myosin are proteins that are present in muscles. Those proteins are helpful in muscle contraction and relaxation.
6. Defensive proteins
Some proteins performed defensive functions. Collagen, lysozyme, and antibodies are proteins that are synthesized in our body to protect the body from foreign materials.
7. Worked as a transport system
Hemoglobin, albumin, protein channels, and carrier protein worked as a transport system. Hemoglobin transports oxygen and carbon dioxide and protein channels and protein carriers move substances across the cell membrane.
8. Maintained pH concentration
Protein is also essential for maintaining pH in the blood.
Factors affecting protein digestion
1. The pH of the digestive system
pH is the most common factor that affects the digestion of proteins. In the stomach protein digestion occurs above 2.0 pH and in the intestine body needs to be approx. 8.0 to 9.0pH this pH is maintained by sodium bicarbonate. It has a favorable pH for the intestinal digestion of protein.
2. HCl
HCl is more important for Protein digestion because HCl denatures complex protein molecules than pepsin, easily cleaved peptide bonds of protein, and activation of pepsinogen to Pepsin is another function of HCl. Pepsin enzymes activate at low pH, this pH is maintained by HCl.
3. Sodium bicarbonate (NaHCO3)
Foodstuff comes in the duodenum from the stomach. Pancreatic juice is released that contains Pancreatic enzymes such as trypsin, chymotrypsin, and elastase which are endopeptidases active at neutral pH. Gastric HCl is neutralized by pancreatic NaHCO3 in the intestine and this creates favorable pH for the action of proteases.
4. Hormones
Hormones also affect the digestion process. Some hormones are involved in the digestion process and initiate enzyme activity. These are
Secretin- It initiates the pancreas to release pancreatic juice. Pancreatic juice contains pancreatic enzymes, without pancreatic enzymes the digestion process does not work.
Cholecystokinin- these hormones initiate the contraction of the gallbladder and bile juice transport from the duodenum.
Enterocrinin- It initiates the intestine to release intestinal juice.
5. Substrate specificity
Enzymes possess a certain degree of substrate specificity in their action.
Example
Trypsin cleaves the peptide bonds, the carbonyl (–CO–) group of which is contributed by arginine or lysine.
Carboxypeptidase B acts on peptide bonds of COOH-terminal amino acid, the amino group of which is contributed by arginine or lysine
6. Dependency on Zn2+
Carboxypeptidases enzymes are metalloenzymes, they are dependent on Zn2+ for their catalytic activity, they are also called Zn-proteases.
Q&A
1. Where does protein digestion begin?
Protein digestion begins in the stomach with pepsin, which is aided by gastric HCl. Pancreatic proteases (trypsin, chymotrypsin, and elastase) and intestinal aminopeptidases, and dipeptidases complete the degradation of proteins to amino acids and some dipeptides.
2. What is the end product of protein digestion?
Free amino and peptides are end products of protein digestion. In the intestine aminopeptidases and Dipeptidases cleave the N-terminal of amino acids and form free amino acids and smaller peptides.
3. Why will a decrease in the pH of the stomach affect protein digestion?
The Pepsin enzyme is responsible for protein digestion. Pepsinogen is the inactive form of pepsin. Pepsin enzymes activate and work at low pH. This enzyme is an acid-stable enzyme, it is optically activated when stomach pH (< 2 pH) decreases therefore decrease in pH of the stomach affects protein digestion.
References
Biochemistry, 4th edition, U. Satyanarayana and U. Chakrapani
Written By: Richa Pachori
