1. Introduction
https://youtu.be/I-EJUlpDpY4
A wide variety of organisms like plants, animals, and microorganisms live on Earth. All these organisms comprise mainly 6 elements viz. carbon, hydrogen, oxygen, nitrogen, sulfur, and phosphorus. there is a balanced cycle between the living and the nonliving world for all the elements. Thus all these elements rotate from the environment to the organism and again from the organism to the environment and the biogeochemical cycle is formed. We are going to discuss the phosphorus cycle in this article which is one of the geochemical cycles in the environment (1).
What is the phosphorus cycle?
- Phosphorus is a very important component of the protoplasm of all organisms.
- It is the structural unit of ADP or ATP and nucleic acids (DNA or RNA).
- Phospholipids made of phosphorus are the building blocks of the bone, cell membrane, and hereditary materials such as RNA and DNA.
Phosphorus is the 15th element of all the elements discovered in nature. This phosphorus element rotates in the environment. That is, phosphorus starts circulating from the physical environment to the living environment and again from the living environment to the physical environment. As a result, there is a cycle formed. This is the phosphorus cycle (3) & (4). This phosphorus cycle has a two-phase
- Terrestrial phase – terrestrial plant→ terrestrial animal→ decomposer
- Aquatic phase – aquatic plant→ aquatic animal→ decomposer
Definition phosphorus cycle
The controlled cyclic process by which natural phosphorus rotates from the environment to the organism and from the organism to the environment and maintains the balance of phosphorus in the environment is called the phosphorus cycle. The phosphorus cycle is the movement of phosphorus through the lithosphere, hydrosphere, and biosphere (1) & (5).
Sources of phosphorus
Phosphorus is a very active element thus this element is not found in Free State in nature as it is easily oxidized. Most of the phosphorus compounds are deposited in the rocks and sediments of the lithosphere. It is found in large quantities as different types of phosphorus compounds or mineral phosphate. The two sources of phosphorus are
- Mineral
- Animal bodies
The main sources of phosphorus in nature are insoluble ferric and calcium phosphates in rocks. Nitric acid produced by nitrification dissolves these phosphates and is transferred to rivers, seas, lakes, groundwater, etc. by erosion and adsorption process. Inorganic phosphate rock is partially composed of apatite. This is also the primary source of phosphorus (2) & (3).
Characteristics of the phosphorus cycle
The phosphorus cycle has some properties.
- The phosphorus cycle is active in both lithospheres, hydrosphere, and biosphere.
- Organic phosphorus present in the dead organism is converted to inorganic phosphate by the bacteria present in the soil. This type of phosphate is absorbed by the tree’s roots.
- Phosphorus is a highly reactive compound. It is found in combination with other elements.
- The amount of phosphorus in the soil is gradually decreasing as the phosphorus in the soil flows into the ocean by water flow.
- Compounds in the phosphorus cycle are mainly in solid state.
- There are different types of phosphorus-rich fertilizers (like ammonium phosphate) are produced from mineral phosphorus compounds.
- Phosphorus in the phosphorus cycle is usually located in nature in the form of orthophosphate ions (PO₄ᶾ-).
- In this cycle, insoluble phosphate is converted into soluble or partially soluble phosphate by plants through soil erosion and rock formation (1) & (5).
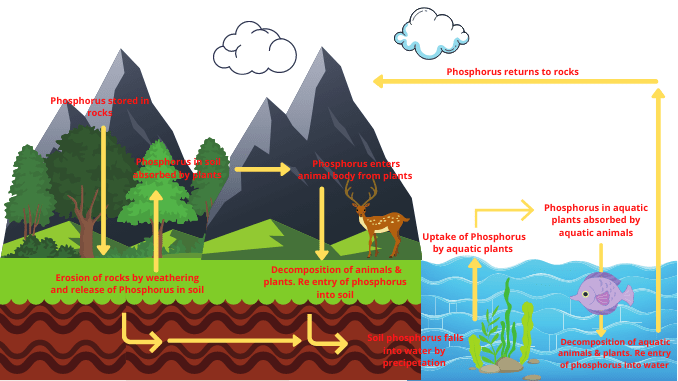
Phosphorus cycle in detail
The phosphorus cycle is considered to be the slowest biogeochemical cycle. Phosphorus is a component of nucleic acids, phospholipids, and various types of phosphorylated compounds.
There is no gaseous phase in the phosphorus. However, it is found in the atmosphere in the form of small particles. These elements rotate from the physical environment to the biosphere and from the biosphere to the physical environment and form the phosphorus cycle. The phosphorus cycle is completed in the following steps.
1. Release of phosphorus in the form of dissolved phosphate
- Rock is one of the primary sources of phosphorus. Extraction of phosphorus occurs from the rocks by weathering.
- Phosphorus exists in the form of insoluble calcium and ferric phosphate in rocks.
- Rock is eroded by precipitation and other sources of erosion and the phosphorus present in the rock is released into the soil.
- Phosphorus is also released into the soil in the form of phosphate from volcanic eruptions and some natural sources.
- This phosphate released from the rock into the soil is dissolved by the nitric acid produced by the nitrification process.
- These phosphates are transferred to the river, seawater, lakes, and groundwater by the erosion and sedimentation process.
- Eventually dissolved phosphates from rivers, lakes, reservoirs, and groundwater are transported to the sea.
- Every year large amounts of phosphate are transported from land to water. In this way, phosphorus flows into the aquatic environment (2) & (7).
2. Use and assimilation of dissolved phosphate
The terrestrial cycle of phosphorus
- Various small and large plants located in the terrestrial environment take this phosphate either directly from the soil or from various bacteria.
- Fertilizers made from inorganic phosphates are often applied to the soil to ensure proper growth of the plant and increase soil fertility.
- This phosphorus enters the body of terrestrial animals from plants through the food chain.
- This element has important effects on the body of animals. It is the structural unit of RNA and DNA, the main nucleic substances in the human body.
The aquatic cycle of phosphorus
- Most of this phosphate in the ocean is stored at the bottom of the sea.
- Plants absorb phosphorus and use it to build their bodies.
- This phosphorus gets transferred from plants to animals via the food chain.
- Aquatic animals use this phosphate for the basic structure of the body.
In this way, the use and assimilation of dissolved phosphate in aquatic environments occur (1) & (5)
3. Return of phosphate from the organism to the environment
Phosphorus returns from the organism to the physical environment in two main ways.
Excretion
- The phosphorus present in the plant body is transferred to the animal body after the animals have taken the plant as food.
- Now, this phosphorus is used by animals for various physiological activities in their bodies.
- Some amount of phosphate remains after the animals use phosphorus in their work.
- That remains phosphate is excreted from the animal’s body through animal excretion and returns to the physical environment.
Released through the decomposition of an organism’s body
- Phosphorus enters the animal’s body through the food chain from the body of the plant. When animals die, the bacteria present in the environment decompose the organism.
- That is phosphatizing bacteria decompose dead organisms after death and convert phosphate from the protoplasm into soluble phosphate.
- In this way, phosphorus is released into the physical environment in the form of phosphate.
Soil-borne organisms decompose plant and animal dead bodies, feces, and urine to produce inorganic phosphate from organic phosphate. This process is called mineralization. This inorganic phosphate is restored in rocks and soils (4) & (5).
Importance of phosphorus cycle
1. Phosphorus is a main component of ATP, the molecule through which energy is stored and transmitted to cells.
2. The element phosphorus is present in the organism in the form of phosphate, which helps in the process of cell division of the organism.
3. Phosphate molecules are present in the constituents of this DNA and RNA.
4. Continuous rotation of this cycle maintains the balance of phosphorus between the organism and the environment.
5. Phosphate is especially needed for the growth of teeth and bones.
6. It is an important component of fertilizers used in agriculture.
7. It plays an important role in the functioning of the kidneys, muscles, and nervous systems.
8. In industry, phosphates are used for a variety of purposes, for example in the food industry it is used as a stabilizer.
9. Phosphorus is a key component in making matches, and fireworks. Similarly, it is used as a detergent as a trisodium phosphate (Na₃PO₄) (5) & (7).
Impact of environment and human activities on the phosphorus cycle
The phosphorus cycle is a balanced natural process but due to human intervention and unplanned activities, the amount of phosphate released into the soil is increasing. Which further results in increasing soil and water pollution. Following human activities causes an imbalance in the phosphorus cycle
1. Excessive deforestation
- Excessive deforestation leads to the extinction of green cover in the environment. Due to this reason, the amount of phosphorus used or consumed by plants or forests in the living environment decreases.
- As a result, a lot of phosphorus remains in the lithosphere and the level of phosphorus is increased in the lithosphere causing a disturbance in the cycle.
2. Agriculture
- To increase food production, excessive amounts of phosphate are applied to agricultural lands as chemical fertilizers. As a result, a lot of phosphorus is mixed in the soil in the form of phosphate and the quality of the soil is lost. Which is one of the causes of soil pollution.
3. Industrial use
- Increasing use of phosphorus in the preparation of matches, fireworks, smoke bombs, etc. As a result, a lot of phosphorus is mixed in the air and pollutes the air.
- Phosphoric acid is formed by reacting with large amounts of phosphorus present in the soil and rainwater. Large amounts of phosphoric acid are carried in water and fall into canals, ponds, lakes, rivers, and oceans. The result is water pollution. Water pollution causes damage to aquatic and terrestrial animals. Drinking polluted water can also lead to kidney infections and decay of bones in humans (4) & (6).
For the above reasons, it is known that the phosphorus cycle is currently being affected in different ways by the environment and humans. As a result, the phosphorus cycle is disrupted.
Finally, the phosphorus cycle is a significant cycle in the environment. This cycle is a very essential biogeochemical cycle for the environment and the organism. Phosphorus cycles are not as complex as the nitrogen and carbon cycles. In the case of phosphorus cycles, the excess of phosphorus is observed in the organic environment rather than in the inorganic environment.
