Introduction
Definition
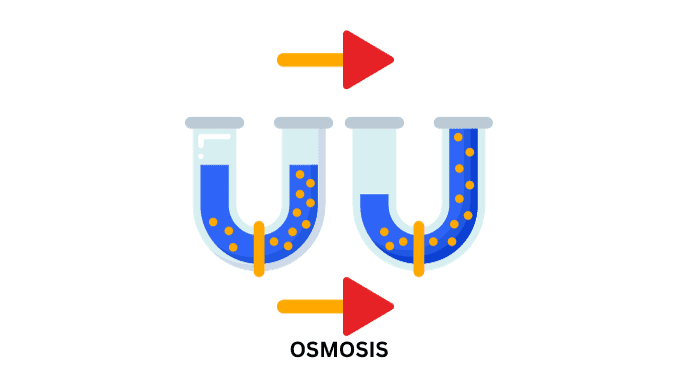
Osmosis is the net diffusion of water molecules from a dilute solution to a concentrated solution when the two are separated by means of a semi-permeable membrane.
Basic Explanation
- When the two solvents having different osmotic concentrations are separated by the means of a semi-permeable membrane, they move from a region of higher chemical potential to a region of lower chemical potential. This movement of the solvent particles (water) is called Osmosis.
- Basically, this involves the diffusion of solvent across a semipermeable membrane in a biological system.
- Pure water has maximum free energy, diffusion dissolves into it, and the free energy of its water molecule decreases.
Importance Of Osmosis
- Cell movement of solvent molecules goes throughout the plant body due to osmosis.
- It plays a key role in the absorption of water by plants.
- The rigidity of plant organs including the shape and form of the organism is maintained through osmosis.
- Leaves become turgid and expand due to their osmotic pressure.
- The opening and closing of the stomata are affected by osmosis.
- Resistance of plants to drought is brought about by the osmotic pressure of their cells.
- Movement of plant and plant parts, bursting of fruits, and sporangia, occur due to osmosis.
Principle Of Osmosis
1. Osmosis is a special type of diffusion of solvent molecules from a low concentration of solution to a higher concentration when separated by a semipermeable membrane.
2. It requires liquid as a medium and the solvent changes from one place to another.
3. The presence of a semipermeable membrane between the two solutions is required.
Osmotic Pressure and Role
The osmotic pressure of a solution is equal to the pressure that must be exerted upon it to prevent the flow of solvent into it across a semipermeable membrane.
The osmotic pressure of a solution depends upon the ratio between the number of solute and solvent particles present in a given solution. Osmotic pressure is measured in Pascals. (1Pa = 1N/m2)
Osmotic Pressure = CST
where,
C = Concentration of a solution.
S = Solution constant = 0.082
T = Absolute temperature [C + 273]
Difference between Hypertonic, Hypotonic, and Isotonic solution
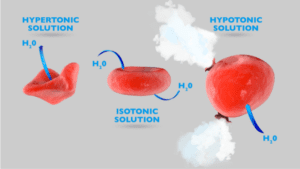
Hypertonic Solution |
Hypotonic Solution |
Isotonic Solution |
| A solution having a concentration such that it gains water by osmosis across a semipermeable membrane. | Having a concentration such that it loses water by osmosis across a semipermeable membrane. | A solution having a concentration such that it doesn’t achieve water by osmosis when separated by a semipermeable membrane. |
| High concentration | Low concentration | Equal solute concentrations |
| It causes cells to shrink | It causes cells to swell | No effects on cells |
| It maintains the quality of the food, thus ensuring food preservation. | It does not support food preservation. | It does not support food preservation. |
| Cells undergo plasmolysis | Cells swell and then rupture | The cells remain the same. |
Cellular osmosis
Mechanism of cellular osmosis
Cellular osmosis is the mechanism through which cells help in regulating their environmental conditions, known as osmotic pressure. It involves the movement of solvent molecules across the cell membranes. It helps maintain a balance between two solutions with different concentrations.
Cellular osmosis includes three basic means of transport:
- Diffusion
- Active transport
- Facilitated diffusion.
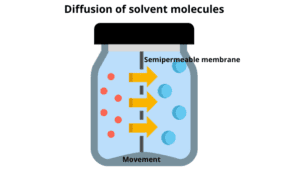
Diffusion
Diffusion can be defined as the net movement of a substance from an area of high to low chemical potential as a result of the kinetic motion of the molecules or atoms. It occurs down the concentration gradient and does not require any carrier molecules.
Active Transport
The transport of solute linked with some metabolic reaction involving expenditure of energy is called active transport. It requires special carrier protein and energy in the form of ATP.
Facilitated Diffusion
It is a spontaneous passage of molecules across a biological membrane mediated by specific transmembrane carrier proteins. It is a kind of passive transport in which the molecules move across the membrane with the help of transport proteins down a concentration gradient.
Role of cell membrane
- The cell membrane has a key role in osmosis that acts as a barrier and allows only selective molecules to pass through it.
- It also helps in regulating the movement of water molecules into and out of cells, thus maintaining an equilibrium.
- The proteins present within the membrane help the movement of the particles against their concentration gradient.
- The membrane keeps the semi-fluid cell contents in place and distinct from the environmental materials.
- It also helps in maintaining the individuality and form of the cell.
Plant cell osmosis
Solvent moves from an area of high concentrations to low concentrations, thus creating a pressure called osmotic pressure that allows movement across the membranes.
The presence of proteins, called aquaporins, helps transport water molecules into and out of cells without ATP. An image representing the movement of water molecules in a plant tissue.
Animal cell osmosis
It is the process by which solvent molecules move across a semipermeable membrane from high concentration to low concentration. It helps maintain a balance in and out of the cell. The animal cell osmosis depends on certain factors like:
- Type of cell and its size
- Temperature
- The concentration of solute inside and outside the cell.
Osmotic pressure also plays a key role in regulating processes like metabolism, growth, and development.
Factors influencing osmosis
1. Concentration
The rate of osmosis reaction depends on the difference between the two solution concentrations. Higher the solute concentration, the lower will be the diffusion ratio of absorbing water through osmosis. At higher temperatures, the diffusion rate becomes higher.
2. Temperature
Higher temperatures result due to higher concentrations and osmosis becomes faster at increasing temperatures.
3. Pressure
Higher pressure results in higher kinetic energy of the molecules that causes the cells to deform.
4. Environmental factors
Environmental factors also play a key role in osmosis.
5. The pressure difference
The pressure difference between two sides of a membrane also affects the osmotic flow between two solutions.
Osmosis in biological systems
Osmosis occurs in biological systems, where the water molecules move from high to low concentration. This process thus helps to maintain a balance within the body.
Osmosis basically deals with the differences in the concentration of the solutes. Greater solute on one side results in the flow of solvent to the other side until it reaches equilibrium. It also plays an important role in maintaining the nutrient balance within cells and tissues.
It enhances diffusion across a semipermeable membrane that allows substances to enter cells without ATP. Helps in facilitating processes like active transport or facilitated diffusion.
Application of osmosis
- In fields including medicine, agriculture, and industry.
- Dialysis treatment is due to the diffusion of small dissolved molecules from one solution through a semipermeable membrane into another.
- Blood is stored in isotonic saline water to avoid hemolysis. Hemolysis is the rupture of red blood cells with the release of hemoglobin.
- Roots absorb soil water by osmosis.
- Resorption of water from kidney tubules through osmosis.
- Desalination of salt water, to reuse it for agriculture purposes.
- Hyperfiltration or reverse osmosis process in water treatment plants.
Q&A
What is a simple definition of osmosis?
It is a special type of diffusion of solvent molecules from a low concentration of solution to a higher concentration when separated by a semipermeable membrane.
What is the perfect definition of osmosis?
Is the net diffusion of water molecules from a dilute solution to a concentrated solution when the two are separated by means of a semi-permeable membrane.
What is an example of osmosis in everyday life?
Roots absorb soil water by osmosis.
What is osmosis and what is it used for?
When the two solvents having different osmotic concentrations are separated by the means of a semi-permeable membrane, they move from a region of higher chemical potential to a region of lower chemical potential.
This movement of solvent particles (water) from one place to another.
It is used for various purposes such as in dialysis treatment and in the desalination of water that can be further used for irrigation purposes.
Summary
- Osmosis is the net diffusion of water molecules from a dilute solution to a concentrated solution when the two are separated by means of a semi-permeable membrane.
- Hence, they move from a region of higher chemical potential to a region of lower chemical potential.
- Pure water has maximum free energy, diffusion dissolves into it, and the free energy of its water molecule decreases.
- Movement of water between cells occurs throughout the plant body due to osmosis.
- Osmosis plays a key role in the absorption of water.
- The rigidity of plant organs including the shape and form of the organism is maintained through osmosis.
- Leaves become turgid and expand due to their osmotic pressure.
- The opening and closing of the stomata is affected by osmosis.
- Resistance of plants to drought is brought about by the osmotic pressure of their cells.
References
https://pubs.rsc.org/en/content/articlehtml/2019/cs/c8cs00420j
https://europepmc.org/article/med/10898241
https://www.sciencedirect.com/science/article/pii/S0301479716308970
https://journals.physiology.org/doi/abs/10.1152/jappl.1962.17.5.841
https://www.jstage.jst.go.jp/article/jjphysiol1950/29/6/29_6_669/_article/-char/ja/
