Introduction
The ion-exchange chromatography is an analytical technique that shows the polarity of the division of ionic and molecular species based on the principles of chromatography. This process is commonly used to separate charged molecules such as proteins, amino acids, etc. Below is a discussion of what is ion-exchange chromatography, its properties, and its functions (2) & (6). It is used to separate and purify mixtures, primarily for synthesis analysis with a detector.
Chromatography is a method of separating chemicals. It has a much higher separation capacity than distillation and extraction. Two major categories of chromatography are liquid and gas chromatography. There are also more different types of chromatography. Ion exchange chromatography is one of them.
Ion exchange chromatography
It is a biological technique that can be used in the separation of ions of similar properties, the analysis of which is rather difficult by many other methods.
This process is defined as a reversible reaction in which free mobile ions of a solid are exchanged for different ions of a similar charge present in the solution. It is a very versatile technique because of its general applicability, good resolution, and high capacity.
When molecules are dissolved in water or other solvents, they break off into charged ions. These ions develop polarity. That means, they can be separated on the basis of polarity. It is a powerful process used for the separation of two proteins. These proteins are very similar to each other but different from each other in having one charged amino acid.
In this chromatography, solutes are located in an ion exchanger due to their inverse interaction with the groups charged in the ion exchanger. Ion exchange chromatography is a type of adsorption chromatography (1) & (5).
Discoverer
The origin of ion-exchange chromatography primarily began between 1935 and 1950. It was introduced by two researchers, Thompson and chemist J. T. Way. When this technique was first developed, it was used for water purification. But in 1935, this analytical process was one of the most common theories.
According to modern theory, this method was introduced by Small, Stevens, and Bauman in 1975. But before 1975, in 1956, Peterson and Sober gave the idea of this theory in order to separate protein molecules. This process is applied to most chemical applications, including distillation, absorption, and filtration (2) & (5).
Types
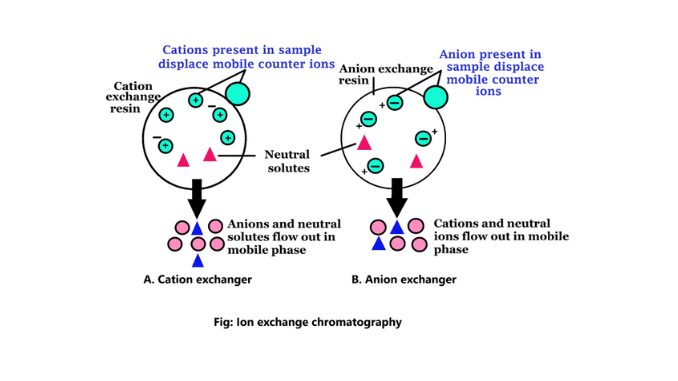
The ion exchanger consists of normally occurring organic biopolymers as an insoluble matrix to which various groups known as fixed ions are covalently connected. These ions are balanced by equal and oppositely charged mobile ions to form the solutions. The solution is known as counter ions. On the basis of counterions, ion-exchange chromatography is used to separate charged molecules. Mainly there are two types of ion exchangers (6).
1. Anion exchanger
Anion exchangers are positively charged, exchangers. These ion exchanges contain negatively charged counter ions. This process is used in the analysis of water, purification of the protein, etc. If a protein has a net negative charge at pH 7.0, it will be attached to an anion exchanger.
There are three types of cation exchangers based on the strength of charged functional groups:
- Strongly basic anion exchanger
- Intermediate basic anion exchanger
- Weakly basic anion exchanger
2. Cation exchanger
They are negatively charged exchangers. These exchangers contain positively charged counter ions. This process is used when the molecule is positively charged. The pH for chromatography is less than pI. So the molecule is positively charged. If a protein has a net positive charge at pH 7.0, it will be combined with a cation exchanger.
According to the strength of charged functional groups, there are three types of cation exchangers:
- Strongly acidic cation exchanger
- Intermediate acidic cation exchanger
- Weakly acidic cation exchanger (2) & (3)
Properties of ion exchangers
There are some general requirements for a suitable ion exchanger that are closely interrelated. Some of the common features of ion exchangers are-
1. The ion exchanger acts as inert supporting media.
2. They are insoluble in water and many organic solvents.
3. Ion exchangers are complex and polymeric types.
4. In this process the ion exchangers are packed in columns to separate the elements.
5. When molecules are dissolved in a solvent, ion exchangers dissociate into charged ions and develop polarity.
6. They are high molecular weight and cross-linked polymers.
7. These ion exchangers contain charged groups that will exchange with other ions in a liquid form in which there is no permanent change in structure.
8. The physical and chemical stability of an exchanger is cross-linking and in contact with the fluid which is influenced by nature (1) & (5).
Principal
- The principle of this method is based on the simple fact that different cations or anions have different capacities to undergo an exchange reaction on the surface of a given exchange.
- The capacity of an ion to undergo an exchange reaction has been found to depend on the exchange and the size of the hydrate ion in the solution.
- Under similar conditions, the capacity has been found to increase with the charge on the ion but has been found to decrease with the increase in the size of the hydrated ion.
- This chromatography depends on the attraction between two opposite charged stationary phases. They are called an ion exchanger and analytes.
- The ion exchanger consists of an inert support medium. This medium consists of positive or negative functional groups.
- Positive of negative charged functional groups are bounded, which will be exchanged with charged ions.
- If the ion exchange chromatography is accomplished, the negatively charged sample elements will interact more with the stationary stage and will exchange like-charged ions already bound to the matrix (1).
The phase of ion-exchange chromatography
There are two phases of this type of chromatography.
- Mobile phase
- Stationary phase
Instruments
Typically, the instruments used in this method are:
- Column
- Suppressor
- Detectors
- Data system
- Pump
- Injector (4)
Process
1. Ion-exchanger is conducted with alkali, then acid to neutralize it. Eventually, it is washed in water. In contrast, the cation exchanger is first treated with acid, neutralized by alkali, and finally rinsed in water.
- The ion-exchanger is packed in a column and aligned with the counterions by passing a buffer of the required pH.
- When samples containing mixtures of compounds are applied onto the top of the exchanger, the solutions having charges similar to that of counter ions get exchanged with the counterions and bind oppositely but strongly to the ion exchanger.
- Other solutions have no compatibility with the stationary phase and are washed with a buffer.
- The pH of the elution buffer is then changed step by step.
- Usually, a buffer with increased pH and ionic energy is applied with an anionic exchanger, while pH reduction with a cation exchanger and increasing ionic energy gradient is applied to a boulder to increase the bound substance.
- The ion exchanger is reproduced after passing the buffer continuously (3) & (6).
Function or uses
It has been widely used for separating many biochemical mixtures such as proteins, amino acids, sugars, nucleic acids, etc.
1. Separation of protein
This process is used for the separation of protein. Proteins are polyionic molecules and most proteins bear a positive charge at acid pH or a negative charge at basic pH. They exchange with one or the other type of cellulose derivative, depending on the pH of the solution. It is selectively eluted from the column by buffers of increasing or decreasing pH.
For example, if a mixture of proteins is placed on carboxymethyl cellulose at pH 4, the proteins are eluted from the column by increasing the pH of the buffer. Selective elution can also be accomplished by using solutions of salts of increasing ionic strength.
2. Separation of sugars
The process was discovered by Khym and Zill in 1951. The sugars are first of all converted into borate complexes. Separation of borate complexes of sugars has been achieved on 11 X 0.9 cm columns of 200- 400 mesh Dowex 1 resin, using a loading of 5 – 10 mg of borate complex and flow rates of 0.5- 1 ml/min. quantitative recovery of sugars is possible after the separation of the borate complexes.
Ion exchange chromatography is also used in many cases.
- This method is used to separate and refine samples of organic compounds with substances such as nucleotides, carbohydrates, etc.
- It regulates the quality of water treatment and deionization and flexibility in solutions, as well as the separation of magnesium and calcium.
- This exchange method has been used extensively in many branches of the industry. Most of these applications are used to measure and analyze residues in pharmaceuticals, including iodides, sulfates, phosphates, as well as potassium and sodium.
- It is used to separate and purify blood components such as albumin, recombinant growth factor, and enzymes.
- This process is used to separate creatine kinase isoenzymes from human serum and tissue sourced in autopsy material.
- It is used in various cases of product development and quality control testing.
- The method identifies and quantifies inert elements used in pharmaceutical formulations.
- This analysis method is widely used in petrochemical, hydrometallurgical, textile, food and beverage, and semiconductor industries among other cases.
- There are various lunar rocks and rare trace elements on Earth. These rocks and elements are analyzed by ion-exchange chromatography (1) & (5).
Advantages
The advantage of using this method is
- This method is a very useful water-softening method.
- One of the advantages of using this method is that it allows easy separation of charged particles.
- It is used for both analytical and preparatory purposes.
- This process is a quick separation process.
- The ion exchange of inorganic ions can be separated by this method.
- Its maintenance is readily available and inexpensive (3).
Disadvantages
- The method allows only charged molecules to be separated. This is one of the major disadvantages of ion exchange chromatography.
- This analytical process has a high-efficiency cost due to the use of a buffer for the separation of components (1).
Q&A
1. What is ion-exchange chromatography?
It is an analytical process that separates ions and polar molecules based on their relation to the ion exchanger. This process is mainly used to separate charged molecules like proteins, nucleotides, amino acids, etc.
2. How does ion exchange chromatography work?
The ion-exchange chromatography works by clearing dissolved ionic contaminants from the water. These ions are interchanged for better ones that won’t demote the property of the water. This process separates molecules based on the net charge at a particular pH.
3. Ion exchange chromatography what elutes first?
When cation exchange chromatography is used, cations will elute out last and the negatively charged molecules will elute out first.
4. What is the principle of ion exchange chromatography?
Molecules separated on the basis of their charge are eluted using different ionic energy solutions. By passing this kind of solution through the column, the molecules finally undergo selective separation according to their individual charges.
5. How to read ion-exchange chromatography graphs?
It is an important technique for the separation of ionic compounds. It is based on ionic interactions between ionic and polar analysis, ions present in the eluent and ionic functional groups are fixed to the chromatographic support.
