Introduction
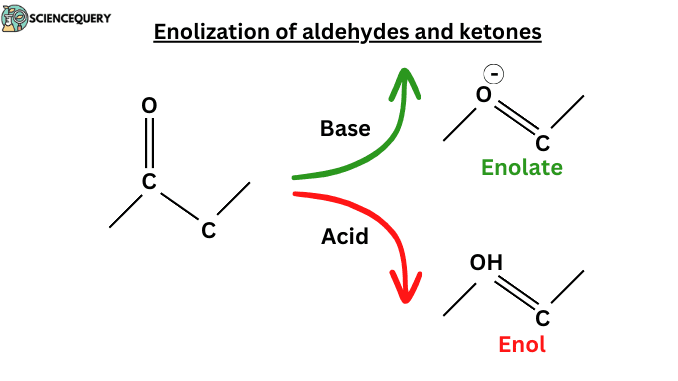
The enol is formed by the transfer of a proton from the central CH2 group of the Keto form to one of the OH groups, a reaction known as enolization. Enolization simply means losing a proton from a Carbon atom and gaining one at Oxygen. The word “enol” is made with two words: ene + ol. “Ene” referred to the C=C double bond and an “ol” referred OH group joined directly to it. Enolization of Aldehydes and Ketones, but of course, enolization is impossible in any carbonyl compound without hydrogen atoms adjacent to the carbonyl group.
Signification of enolization
- Enols and enolates are formed through enolization. In enolization, ketones and aldehydes convert into enols or enolate ions.
- Enolate is the common conjugate base of both the keto and enol forms. Enolate ions are useful in organic synthesis.
- They are involved in the formation of carbon-carbon bonds because they are a major source of carbon nucleophiles.
Role of aldehyde and ketones in enolization reactions
- In enolization aldehyde, ketone, and esters are converted to their corresponding enols.
- Aldehyde and ketone play important roles in enolization because both are the type of carbonyl compound (containing the carbonyl group (C=O) group).
- They have an α carbon atom which is attached to hydrogen. It’s called α hydrogen. This α-hydrogen is responsible for the enolization.
- A carbon atom that is directly attached to a carbonyl group (C=O) is called α carbon.
1. Enolization reaction mechanism
- The formation of enols is called enolization.
- Enolization is a slow process in a neutral solution.
- It is an acid or base-catalyzed reaction. Catalyzer increases the reaction rate.
- In their acid is used as a catalyst. The compound that provides H+ ions is known as acids like H2SO4, HNO3, HCl, etc. In water, acids exist in the form of hydronium (H3O+).
- The mechanism consists of two stages: The Enolisation mechanism differs in acidic and basic medium. These are the following steps of enolization.
- For the formation of enol, we need carbonyl compounds that have α-hydrogen.
- A lone pair electron of oxygen attacks on hydrogen atom of hydronium and takes hydrogen to form the OH group and H2O molecule. The formed compound is called carbocation.
- Now oxygen is positively charged. This reaction is called protonation. This step is more than to second step.
- In the second step, lone pair electrons of water molecules attack CH3 and remove hydrogen to stabilize the carbocation.
- Then a double bond will be pulled toward itself and form enol and hydronium ions again.
Factors affecting enolization
1. Temperature
Temperature affects the enolization. A high temperature range reduces their hydrations and makes them unstable thereby they dissociate into smaller aggregates.
2. Influence of solvent
If the solvent is polar it increases the percent of keto forms. Polar solvent, which is capable of forming hydrogen bonding, will make lone pairs less available for intramolecular H-bonding, hence % of keto increases.
Enolization of aldehydes
Enolization reactions specific to aldehydes
The Enolization of aldehyde is catalyzed by an acid or base catalyst.
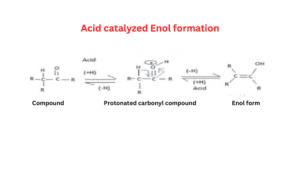
Acid-catalyzed enolization of aldehyde
In the acid-catalyzed reaction, the molecule is first protonated on oxygen and then loses a proton from C in a second step.
Base-catalyzed enolization of aldehyde
- In the base-catalysed reaction the C–H proton is removed first by the base, say a hydroxide ion, and the proton is added to the oxygen atom in a second step.
- In this condition, aldehyde acts as an acid donating proton from α carbon to base. Then an enolate ion is formed which is stabilized by resonance.
- Oxygen is negatively charged. Negatively charged oxygen reacts with HOH and forms enol.

Enolization mechanism in an acidic medium
- In an acidic medium, H+ is present which requires electrons in this condition.
- Oxygen of ketone reacts with hydrogen of hydronium ion which is present in the solution.
- Oxygen donates its lone pair electron which gives a positive charge to oxygen.
- If Oxygen has a positive charge it is a very unstable form because it is a highly negatively charged compound.
- To gain a stabilized form resonance takes place and a double bond will be pulled toward itself.
- CH3 will donate hydrogen to form carbocation or protonated keto formed and it also stabilizes the carbocation then enol is formed.
- In step 1 bond formation takes place, in the second step resonance takes place then the sigma bond breaks and a new bond is formed
Enolization mechanism in basic medium
- In a basic medium, OH ion is present which removes α- hydrogen from the ketone and forms an alkene pi bond.
- This process is called deprotonation and the compound name is enolate.
- Resonance stabilizes the enolate ion and oxygen becomes negatively charged.
- It reacts with HOH to form enol. This process is called protonation.
Summary
- Compounds that have both double bonds and alcohol groups are known as enol and enolization is the process of enol formation.
- In enolization, ketone and aldehyde convert into corresponding enols.
- The enolization process takes place in the presence of an acid or base catalyst.
- In the acid-catalyzed enolization, the first protonation occurs on oxygen, and in the second step, deprotonation occurs.
- In the base-catalyzed reaction, the first step is deprotonation and the second step is deprotonation.
- Enolization is important for organic synthesis. It’s involved in the formation of carbon-carbon bonds.
References
Organic chemistry, 2nd edition, Jonathan Clayden, Nick Greeves
Organic Chemistry by Robert C. Neuman, Jr.
Written By: Richa Pachori
