Introduction
Anion exchange chromatography is a method of ion-exchange chromatography. This method is used to separate molecules according to their net surface charge.
The ion-exchange chromatography is an analytical technique that shows the polarity of the division of ionic and molecular species based on the principles of chromatography. This process is commonly used to separate charged molecules such as proteins, amino acids, etc.
Chromatography is a method of separating molecules. Ion exchange chromatography is a process that can be used in the separation of ions from a chemical. When molecules are dissolved in some solvent, they break off into charged ions. These ions develop polarity. That means they can be separated on the basis of polarity.
Basically, there are two types of ion-exchange chromatography, anion-exchange chromatography and cation-exchange chromatography (1) & (3).
Anion exchange chromatography
Definition
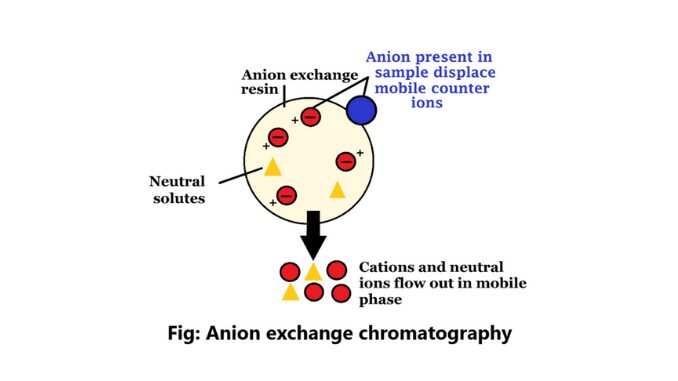
Anion exchange chromatography is mainly a method that isolates the elements according to the charges using an ion-exchange resin including positively charged groups.
Properties
1. It is a biotechnical method.
2. This chromatography is mainly used for the separation of ions from a substance.
3. It is commonly used to purify amino acids, proteins, carbohydrates, etc.
4. This process can separate a large type of molecule.
5. It will bind the negatively charged molecules.
6. It is a measurement of the number of negative charges that can bind an exchange resin and is reported as the equivalent of a single charged ion per gram of resin (2) & (4).
Anion exchanger
Anion exchangers in this process are positively charged exchangers. Purification in protein is complete through this process. If a protein has a net negative charge at pH 7.0, it will be attached to an anion exchanger. The ion exchanges in anion exchange chromatography contain negatively charged counter ions (4).
Types of anion exchanger
Anion exchangers in anion exchange chromatography are basically three types. They are divided based on the strength of charged functional groups. These are
- Strongly basic anion exchanger
- Intermediate basic anion exchanger
- Weakly basic anion exchanger (4).
| Type | Matrix | Functional groups | Name of functional groups |
| Strongly basic anion exchanger | Cellulose
Dextram polystyrene |
CH₂N⁺(CH₃)₃
CH₂CHN⁺ |
Trimethylaminomethyl
Trimethylaminoethyl. |
| Weakly basic anion exchanger | Agarose
Cellulose Dextram Polystyrene |
CH₂CH₂ N⁺H3⁻
CH₂CH₂N⁺H(CH₂CH₃)₂
|
Aminoethyl
Diethylaminoethyl |
Principal
- In anion exchange chromatography the charge on the net surface of a protein varies with the pH in a way that is determined by the isoelectric point or pI of the protein.
- At a pH equal to the pI of the protein, the protein does not carry any net charge.
- In this ion-exchange chromatography, if the pH is below the pI, the protein carries a net positive charge.
- If the buffer pH level is raised above the pI of a protein, it also carries a net negative charge.
- Since the pI of a protein is determined by its primary amino acid sequence and so it can be calculated.
- The positively charged ion exchange resin is chosen when the protein carries a net negative charge in the pH.
- Different pI standard proteins will have different degrees of charge at a given pH and thus have different relationships for positively charged surface groups in anion exchange media.
- At an individual loading buffer pH, all properly charged proteins will bind to the resin.
- The proteins in anion exchange chromatography will be arranged in a sequence depending on their net surface charge (1) & (6).
Function or uses
1. The primary function of anion exchange chromatography is to purify proteins, amino acids, and some acidic solutions.
2. Anion exchange chromatography has positively charged groups that will attract negatively charged anions. These are known as basic ion exchange materials.
3. This method uses some preparative and analytical processes.
4. Combined with optimized binding, anion exchange chromatography can be used in some stages of biomolecule isolation, separation of analytes, and removal of contaminants such as endotoxins, host cell proteins, etc.
5. Anion exchange chromatography is important for all types of buffer pH and ionic energy.
6. This method is used in many branches of the industry. Most of these applications are used to measure and analyze residues in pharmaceuticals, including iodides, sulfates, phosphates, as well as potassium and sodium.
7. Anion exchange chromatography is used to separate the complex mixture of 18 amino acids obtained by the acid hydrolysis of proteins.
8. This type of ion exchange chromatography is mainly used to isolate the charged molecules.
9. Water crosses these exchange sites through a device that aids in ionization.
10. This process is a very important tool to exchange or separate contaminants in low concentrations.
11. Anion exchange can help in identifying the ion exchange which is very effective for treatment (1) & (5).
Q&A
1. How does anion exchange chromatography work?
It is a process that uses ion-exchange resins to separate substances based on their charge using positively charged groups, such as diethyl-aminothyl groups. In this chromatography, the resin is coated with positively charged counter ions.
2. What is anion exchange chromatography?
A process of ion-exchange chromatography that isolates the elements according to the charges using an ion-exchange resin including positively charged groups.
3. How to separate amino acids using anion exchange chromatography?
A mixture of some amino acids is isolated in a strong acid ion-exchange chromatography. The amino acids are separated by sodium citrate buffer and they are displaced by changing the pH of the buffer solution as a function of their pI values.
4. How to make sharp peaks in anion exchange chromatography?
In this, the chromatogram is a graph. It indicates the signal in the detector over time. As the substances are found by the instrument, the symbol increases and the chromatogram shows a “peak”. Gradients and fast flows will work for sharp peaks. Higher temperatures and smaller particle diameters will aid in the formation of sharp peaks.
5. How does high-performance anion-exchange chromatography work?
High-performance ion exchange chromatography is a powerful analytical tool for carbohydrate sorting by size, composition, anomaly, and structural properties, resulting in the ability to isolate alditols, amino sugars, monosaccharides, polysaccharides, and oligosaccharides linkage isomerism.
Reference
1. B. Powar and G. R. Chatwal. Biochemistry, B. SC (general & honors course) and M. Sc. Himalaya publishing house, Chapter: Biochemical Technique. Page no: 97 to 99.
