Introduction
The biuret reaction is a chemical test for proteins and polypeptides. This test is used for all compounds containing two or more peptide bonds. There are various types of biochemical compounds in nature. Biuret is one such biochemical compound. It is a chemical substance that is used for the identification of proteins and peptides.
Biuret is formed by heating urea to 180°C. This biochemical compound is mainly used in the laboratory. It is mainly a member of the class of condensed urea which is the compound formed by condensation of two molecules of urea.
Molecular formula of biuret is C₂H₅N₃O₂. Biuret is a white solid substance. It is soluble in hot water.
The word “biuret” describes an organic compound family that has (HN- CO-)₂N- group. This compound may be used to test the aqueous sample. So Biuret is a very important chemical substance. Below is a discussion of the biuret reaction (4) & (5).
What is a biuret reaction?
A Biuret reaction is a chemical reaction that creates a purple color when most protein or biuret is revealed to be copper sulfate in an alkaline solution. It is a general test for protein (2).
Interesting features of the biuret reaction
- This reaction is also known as Piotrowski’s test.
- The reaction is named biuret reaction because this reaction gives a positive reaction to the peptide bonds in the biuret molecules.
- When biuret is reacted with dilute copper sulfate in an alkaline medium, a purple color is produced.
- This reaction is performed on compounds containing two or more CO– NH groups, i.e. peptide bonds.
- All peptides that have at least two peptide links give a positive biuret reaction.
- Color of biuret reaction due to the formation of a copper coordinated complex.
- A biuret reagent can be used to test aquatic solutions.
- The presence of magnesium and ammonium ions interferes with the biuret reaction. This can be out by using excess alkali (2) & (4).
Basic Principle
- A Biuret test is a chemical substance that determines the presence of peptide bonds in an element.
- When the biuret is reacted with dilute copper sulfate, a violet substance is formed in the presence of alkalis. The reason for the violet color of the sample is the formation of a chelate complex of the copper alloying complex.
- Because the peptide does not share a pair of electrons in nitrogen and oxygen of water, the blue copper II ions form a complex with peptide bonds. This reaction occurs in the presence of alkaline solutions.
- A color combination complex is generated between copper ions and carbonyl oxygen and amide nitrogen of the polypeptide chain.
- When the complex is produced, the color does not change. That means, the complex turns from blue to purple or violet.
- The peptide complex’s number depends on the thickness of the purple color. As the color thickness increases the number of peptide complexes also increases. And if the color thickness decreases, the number of peptide copper complexes also decreases.
- Depending on the principle of this reaction, peptide bonds can be found in any biological fluid.
- Biuret reaction occurs in a compound that has at least two, H₂N–CH₂-, H₂N–C, and H₂N–CS- similar groups directly attached or by a nitrogen or carbon atom.
- A biuret reagent is a solution consisting of potassium hydroxide, hydrated copper II sulfate, and potassium sodium tartrate.
- Blue copper II ions are basically connected to six adjacent peptide bonds (3) & (6).
Purpose
The main purpose of this reaction is
- This reaction is used to identify the presence and concentration of protein in a sample.
- And displays the presence and number of peptide bonds in any chemical sample.
- This method gives a positive result for testing peptide bonds. So this reaction is also used in the laboratory (2).
Requirements
The materials that are required in biuret reaction are-
- 5 % albumin and 1% alanine.
- Dry test tubes.
- Deionized water.
- Water bath.
- And biuret reagents. These reagents are made of sodium hydroxide, copper sulfate, and sodium-potassium tartrate (1) & (5).
The reaction of Biuret reaction Procedure
- To do this test, first, take 3 dry and clean test tubes.
- Then 1 to 2 ml of test solution, albumin, and deionized water should be taken in each test tube.
- 1 to 2 ml of biuret reagent was then added to the solution in each test tube.
- Then take the solution to mix well and keep it for 5 to 7 minutes.
- Finally, it can be observed that the solution is changing color (1) & (2).
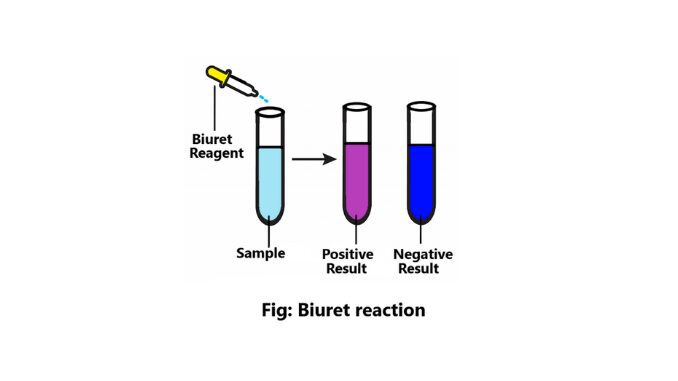
Reaction result
- If no color change is observed in the sample i.e. blue color is present then it is to be understood that no protein is present in the sample.
- If the solution is blue to purple or violet then it is known that the sample contains protein.
- And even if the sample is blue to pink, it is seen that peptide is present in this solution.
Positive result
- The color changes i.e. from blue to purple or violet.
- All proteins and peptides give positive results.
Negative result
There is no change of color in this sample (2).
Uses
Biuret reaction is a very important method. But many times magnesium and ammonium ions interfere with this reaction. This barrier can be overcome by using an excess amount of alkali. It is used in different cases.
- The pure protein content of skimmed milk, as well as the protein content of whole milk, can be measured with the biuret reaction.
- This process is used to detect the protein amount in urine.
- Biuret reaction is used for the quantitative determination of total protein using spectrophotometric analysis (3).
Advantage
- This is the easiest and quickest way to identify a protein in a sample.
- It gives a specific color to the sample which proves the presence of protein in the sample.
- This method does not cause deviations like other methods for protein or peptide identification.
- Biuret reaction identified N from proteins. And non-protein nitrogen is not identified by this test (4) & (5).
Disadvantage
- At least 2-4 mg of protein needs to be identified for this test.
- Sometimes the high concentration of ammonium salts affects the reaction.
- Biuret reaction provides different colors for different types of proteins such as gelatine generates a pink-purple color.
- This reaction has low sensitivity (3).
Q&A
1. Which color choice represents a positive reaction for the biuret test?
The violet color represents a positive reaction for the biuret test.
2. Which statement about the biuret reaction for total protein is true?
Protein compounds and polypeptides with repeating imine groups react.
3. What can falsely elevate serum total protein levels when using the biuret reaction?
The biuret reaction is used to identify the total protein content of serum. This reaction is a widely used method of measuring serum protein. The serum protein reacts with copper sulfate in sodium hydroxide to form a violet biuret complex.
4. Which of the following best describes a positive reaction in the biuret test”?
Violet color describes a positive reaction.
5. Which of the 4 macromolecules will biuret reagent give a positive reaction to?
Egg albumin plus biuret reagent is a positive control for the protein test. It displays a positive reaction result.
