Introduction
Amino acids are ionized in an aqueous solution. Those alpha-amino acids having a single amino group and a single carboxyl group crystallize from neutral aqueous solutions as fully ionized species, known as a zwitterion. It is an inner salt. It is mainly caused by an aqueous solution of amino acids.
The –COOH and -NH₂ groups are ionized groups. These two groups of amino acids act as proton donors or proton acceptors. For this reason, amino acids are called ampholytes. At a specific pH, the amino acid carries both charges (positive and negative) and exists as a zwitterion.
In an acid solution, amino acids carry positive charges and they move towards the cathode in an electric field. And in an alkaline solution, they carry negative charges and move toward the anode. Amino acid exists as zwitterion under the condition. This ion is very important. Its structure and function are discussed below (4) & (5).
Zwitterion
Meaning
The word zwitterion comes from the German word “zwitter”, which means hybrid. So it is also known as hybrid ions.
Definition
A molecule or ion that individually produces positive and negative charged groups. This molecule is called a zwitterion. It is mostly electrically neutral. They are also known as inner salt or dipolar ions. All protein molecules consist of amino acids that carry –COOH and -NH₂ groups in their side chains.
These groups contain both acidic and basic groups. They can react with both acids and alkaline to form salts. The carboxyl group is acidic. It can donate a proton by dissociation. The amino group is basic and can accept a proton. When amino groups and carboxyl groups are ionized, the amino acids are known as zwitterion (1) & (2).
Features
Amino acids are the building blocks of protein and contain both acidic and basic groups. Both of these groups donate and accept protons. Acidic and basic groups of amino acids are ionized and form this ion. There are some features of this ion.
- These ions act as proton donors and proton acceptors, so they are ampholyte types.
- It has two functional groups as amino group and the carboxyl group.
- They are often soluble in water.
- The amino group of these ions contains a positive charge and the carboxyl group contains a negative charge.
- Its net charge is zero.
- They are called dipolar ions because they have negative and positive ends.
- It does not shift to the anode and cathode during electrolysis.
- These ions act as both acids and bases.
- The carboxyl group of these ions is a proton donor and acidic type.
- This ion has an isoelectric point.
- They are not neutral in a pure aqueous solution, because the acidic and basic forms of this ion are different (3) & (6).
Structure
It is mainly a form of amino acid. There are two groups present in this ion. These are the amino group and the carboxyl group. The amino group is positively charged and the carboxyl group is negatively charged. The groups are both acidic and alkaline. In acid and base solution, the amino acid forms salts. This is a zwitterion.
The amino acid exists as zwitterion when they are ionized. This theory is known as zwitterion theory. The ions exist as double-charged molecules and are electrically neutral.
When amino acids are in zwitterion form, they will not be transferred to electrodes in an electric field. This ion has an isoelectric point. The isoelectric point is the pH value at which the amino acid has no tendency to shift either to the positive or negative electrode in electric fields.
Under normal temperature, in the cell, the amino group is a proton acceptor because the pKa of this group is close to 9. The carboxyl group is a proton donor. The pKa of the carboxyl group is below 3. So the pH range of 6.8 to 7.4 amino acids is a zwitterion. This ion is the form of amino acids that exist in the solid state (5).
Function
- These ions are very useful ions. They are used in medical and biological fields.
- Polymers of Zwitterion are widely used to obstruct aquatic organisms from building up on boats in marine regions.
- This ion is used to isolate protein molecules by their molar mass.
- They are also used in drug delivery, medical implants, blood contact sensors, separation of the cell membrane, etc.
- It is used in the formation of food dye.
- The ions are used to make antioxidants in the body. It produces a powerful antioxidant that helps to protect the cell from oxidative damage caused by free radicals.
- It provides strength to the muscles.
- This ion has an effective role in human metabolism.
- Some zwitterion is used to replicate cell pellets (4) & (6).
Examples with structures
Mainly all the amino acids have two functional groups amino and carboxyl groups. Among these two groups, amino groups act as a base and the carboxyl group acts as an acid. An amino acid has these properties because of its pH value. So all the amino acid molecules which are ionized, exist as zwitterions. Here are some examples of zwitterions (4).
1. Glycine
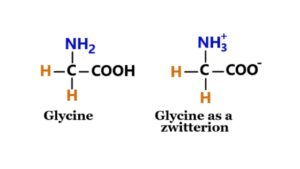
It is an amino acid having a single hydrogen atom in its side chain. Glycine is a stable amino acid. Its formula is NH2-CH2-COOH. They are used to create proteins in the body (6).
2. Betaine
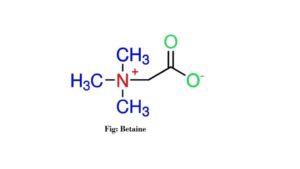
Betaine is an amino acid derivative. Its chemical formula is C5H11NO2. This ion is soluble in water. Its melting point is 180°C. Betaine is a methylated group of amino acids. It is a special type of zwitterion. The ion is also known as trimethylglycine. It is used for the treatment of homocystinuria (2).
3. Sulfamidic acid
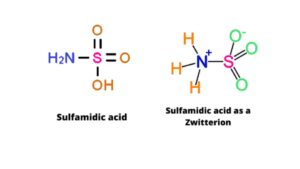
Sulfamidic acid is a colorless compound and is soluble in water. The molar mass of Sulfamidic acid is 97.10 gm/mol. Its chemical formula is H3NSO3. This acid is mainly used for removing rust limescale. It is also used in electroplating, cleaning metal, and stabilizing chlorine. Sulfamidic acid consists of a single sulfur atom with a single bond to the hydroxyl and amino groups and a double bond to two oxygen atoms (5).
4. Threonine
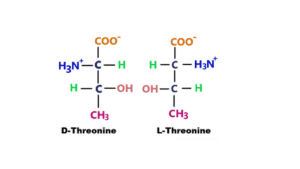
Threonine contains an amino group, a carboxyl group, and a side chain with a hydroxyl group. It is used in the biosynthesis of proteins. This ion is synthesized from E. coli bacteria. The chemical formula of threonine is C4H9NO3. Its molar mass is 119.12 gm/mol. D-threonine and L-Threonine both are active forms of threonine. They play an important role in the metabolism of saccharomyces cerevisiae (4).
5. Alanine
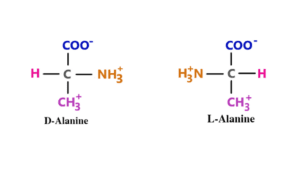
Alanine is the common residue for protein synthesis. Its chemical formula is C3H7NO2. This ion is an essential source of strength for muscles and nerve systems. It is naturally formed in the human body. L-alanine transfers a proton from the carboxyl group to the amino group. Both groups are attached to the central carbon atom of alanine. The carbon atom carries a methyl group (4).
Q&A
1. What is a zwitterion?
A zwitterion is an ion or molecule with a positive and a negative electrical charge. An amino acid contains acidic and alkaline groups. These groups both can donate and accept protein.
The carboxyl group is acidic and can donate a proton. On the other hand, the amino group is basic and can accept a proton. When both amino and carboxyl groups are ionized, the amino acid is called a zwitterion.
2. Which of the following is a zwitterion?
Alanine is a zwitterion. When Alanine is dissolved in an aqueous solution, then zwitterion is formed.
3. Which structure represents a zwitterion?
Amino acid structures represent a zwitterion. They are made up of an amino group and a carboxyl group. The amino group contains a positive charge and the carboxyl group contains a negative charge. Twenty different amino acids are found in proteins. They share the structure of zwitterion. Here R represents one of the 20 possible side chains on an amino acid.

