Introduction
Serial dilution is a microbiological process where a substance is stepwise diluted in a solution. This serial dilution method is a technological method that is used in the isolation of microorganisms. There are two types of dilution.
- Simple dilution
- Serial dilution.
Below is a discussion of what the serial dilution method is and how it’s to be calculated (1).
Let us see the basis of the solution, that is solute, and solvent before discussing serial dilution.
The solution is a mixture of two or more elements in a solvent. It can be prepared by dissolving solutes in solvent or diluted in a diluent, e.g. water, alcohol, etc. Usually, the same solvent or diluent is used for the dilution of the solution to prepare a working solution (1).
Serial dilution is a biotechnical method. In microbiology, isolation is defined as the separation of a strain from a natural or mixed population of living microorganisms. Soil is a heterogeneous mixture of various types of microorganisms including bacteria, actinomycetes, fungi, algae, and protozoa.
These microorganisms play an important role in soil properties. Isolating bacteria from the soil is an important first step in many experiments. Once they are isolated, they can be analyzed to determine things, such as their species and their function in the soil.
Several techniques are used for isolation. Serial dilution is one of them. Another technique is isolation on solid medium or isolation by plating media, single cell isolation using micromanipulation, & selective methods, like the use of chemical agents, enrichment culture, selective adjustments of pH of the growth medium, etc. (2).
Definition
Serial dilution is a biotechnological process that describes how to decrease the concentration of a substance or an element in a solution by repeatedly diluting it with a solvent (1).
Description
Serial dilution is dilution whereas the concentration decreases by the same quantity in each stage.
It is a laboratory process. Here a stepwise dilution process is performed on a solution with an added liquid component.
Serial dilution is multiplicative of the concentration. The objective of the serial dilution method is to estimate the concentration of an unknown sample by counting the number of colonies cultured from serial dilution of the sample, and then backtracking the measured counts to the unknown concentration.
This biotechnological method is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration.
Each dilution will reduce the concentration of microorganisms by specific amounts. In this type of dilution, both solid and liquid samples are used as stock (3) & (5).
Testing of solid samples by serial dilution method
- Weigh 1 g of soil and homogenize with the help of pestle-mortar.
- Transfer into a 9 ml after blank and vortex for a few minutes.
Testing of liquid samples by serial dilution method
- Transfer 1 ml liquid into 9 ml water blank and vortex.
- From the stock solution, 1 ml is transferred to 9 ml water blank and this dilution will be 10⁻¹
- Further dilution and so on at times.
This method is basically used for bacterial counts. Sometimes it can be used for fungi and virus counts. Serial dilution is a series of dilution steps to convert a contracted solution to a more usable concentration.
Here, cell number or density gradually decreases as the sequence number increases at each step. Robert Koch invented this method in 1883. He published the identification method for microorganisms in water.
Serial dilution has many practical applications but has some limitations. These processes occurred step by step, so it required more extended time (1) & (5).
Example
One example of serial dilution is the use of tea or coffee in daily life. A certain amount of cold-press coffee is added to the coffee. And water is added to it to get the desired consistency of coffee (1).
Uses
- Serial dilutions are often used in laboratory work to prepare a chemical or biological sample with a concentration of an element.
- This method is used in laboratory work, including microbiology and biochemistry experiments.
- Serial dilution methods are used to obtain a proper measure of the number of bacteria or other microorganisms in a sample.
- Pharmaceutical companies test the purity of a drug using the serial dilution method.
- Samples must be prepared to test the concentration of pollutants in water or soil. Serial dilutions are used in the environmental analysis of these samples.
- A hospital laboratory collects a sample of the patient’s blood to test the glucose concentration. A serial dilution method can be used to prepare this sample.
- This technique is used in various fields to prepare accurately measured dilute solutions of serial liquids.
- It helps reduce usable cell size to lower densities (1) & (4).
Calculating serial dilutions
The dilution factor for a single tube in a set is:
Volume of sample/ Volume of sample + Volume of diluent.
For example, 1 ml of sample is added to 9 ml of diluent. In this case, the dilution factor is
1 ml/ 1 ml+ 9ml= 1/10= 10⁻¹
After the first tube, each tube is a dilution of the previous dilution tube.
So Total dilution factors are – Dilution of the first tube * dilution of the second tube
Dilution of the first tube is 10⁻¹
And dilution of the second tube is also 10⁻¹
So total dilution factor is 10⁻¹* 10⁻¹= 10⁻² (2).
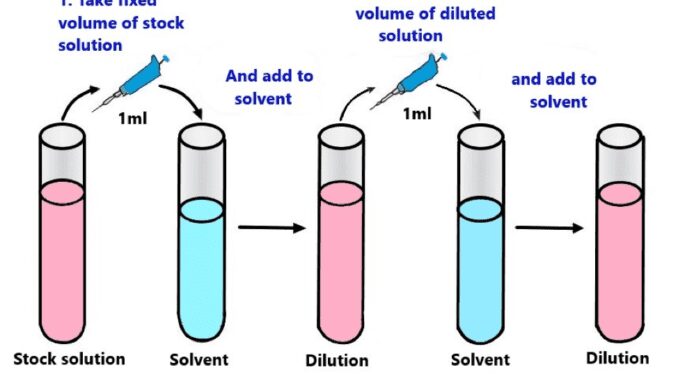
Diagram
Calculation formula
- The dilution factor of each tube in a set is
Volume of sample/ volume of sample+ volume of diluent
- The formula for the total dilution factor
Dilution of first tube * dilution of the second tube
Closing summary
- The serial dilution method is used to dilute a stock solution to prepare a working solution of the desired concentration.
- Is more complex than the simple dilution method.
- This method is used for bacterial enumeration, cell enumeration, etc.
- Serial dilutions are a more accurate method. The accuracy obtained by serial dilution is higher than that of the simple dilution method.
- If one needs to dilute a bacterial culture 10,000 times from a bacterial stock, one can use the serial dilution method.
- The most convenient method is to follow the 10-fold dilution principle.
- To prepare a serial dilution the desired number of tubes must be used to obtain that fluid.
- These dilutions can be done in a stepwise manner.
- Tube sizes for serial dilutions should be determined based on liquid volume.
- In this case, we will dilute 10 fold to a final volume of 10 ml so we may need to use 15 ml tubes and liquid media.
- Here the dilution factor at each stage is constant.
- It is a process of sequential dilutions used to reduce a dense culture of cells to a more usable concentration.
- In this process, a solution is diluted step by step.
- Depending on the estimated concentration of the organism in a sample, the amount of dilution is determined. As an example, if a water sample is taken from a highly polluted environment, the dilution factor is increased and a dilution factor may be sufficient in a less polluted water sample (1) & (2).
Q&A
1. Calculating serial dilution?
In serial dilution, at first, multiply the dilution factors for each step. Then the dilution factor or the dilution is the initial volume divided by the final volume.
Serial dilution= final volume (diluent volume+ stock volume)/ volume of stock transferred.
Serial dilution is done by this formula.
2. Why serial dilution is important
Serial dilution is used to reduce a dense culture of cells to a concentration level that is more usable. This method is also used in both chemistry and biology.
3. What is serial dilution method?
Serial dilution is a microbiological method that is the stepwise dilution of an element in a solution.
4. When are serial dilutions used?
These methods are used to calculate the concentration of microorganisms.
5. Why are serial dilutions more accurate?
Serial dilution is more accurate because it is used to describe how to decrease the concentration of a substance or an element in a solution repeatedly.
6. Are serial dilutions more accurate?
Serial dilution methods are more accurate because they are used to reduce a dense culture of cells to a more usable concentration.
7. Which set of serial dilution plates shows a successful dilution?
The Spread plate shows a successful dilution.
Written By: Manisha Bharati
