Know in one minute about molar conductivity
|
Introduction
Welcome to all science enthusiasts, in this article we will discuss one of the significant topics in physical chemistry which is Molar Conductivity.
Further going through the complete article we will get familiar with topics like limiting molar conductance, unit, formula, and variation in molar conductance.
Molar Conductivity
It refers to the conductivity of an electrolyte in a solution when 1 mole ( 6.022 X1023 ions) of electrolyte is dissolved in a solution.
Represented by λm (lambda).
The formula for Molar conductivity
λm = κA/ l
λm = κ ✕ V ( when length = 1 and hence A=V)
Where κ (kappa) is conductivity.
A is an area of cross-section of electrodes
l is the distance between electrodes and,
V is a volume of solution (1).
Unit
The general S.I. unit is S.m2.mol-1 (Siemens × meter square per mol).
Additionally, it also has some other SI units like,
- S m-1 L mol-1 (Siemens liter per mol per meter).
- MilliSiemen ( 10-3 S).
The molar conductivity is different for different types of elements.
S.No. |
Ions |
Molar Conductivity (*10-4 ) |
| 1. | Potassium (K+) | 73.5 |
| 2. | Nitrate(NO3–) | 71.4 |
| 3. | Hydroxide(OH–) | 198.6 |
| 4. | Sodium(Na+) | 50.1 |
| 5. | Ammonium(NH3+) | 73.5 |
| 6. | Lithium (Li+) | 38.7 |
| 7. | Silver(Ag+) | 69.1 |
| 8. | Bromide(Br–) | 78.1 |
| 9. | Iodide(I–) | 76.8 |
| 10. | Chloride(Cl–) | 76.3 |
| 11. | Fluoride(F–) | 54.4 |
Variation in Molar Conductivity
It is a property of electrolytes and it varies from one electrolyte to another. Thus, depends on the concentration of electrolytes.
When concentration increases molar conductivity decreases and vice versa.
It also depends upon the following factors :
- Nature of Electrolyte – the number of ions is proportional to conductivity.
- The concentration of the solution – when dilution increases, conductivity increases.
- Temperature – rise in temperature increases conductivity.
Moreover, the concentration of electrolytes plays an important role in the variation of molar conductance. Let us understand the variation with the help of a graph.
Note: units in the graph are – for Λm S.cm2.mol-1 and for concentration (mol/L)1/2
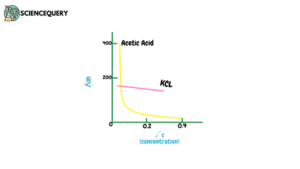
In this graph, we can see that Acetic acid being a weak electrolyte shows a sudden increase in molar conductivity when it is further diluted, while KCl (potassium chloride) is a strong electrolyte but still shows a very minimal increase.
This happens because on dilution the total volume V of a solution containing one mole of electrolyte increases (1).
Molar Conductivity of NaCl (Sodium Chloride)
For this, we will take the molar conductivity of the two ions from the salt (Na+ and Cl-) and will take their sum.
λ∞m NaCl = λ∘Na+ + λ∘Cl
Thus, λ∞m NaCl = 50.1+76.3
λ∞m NaCl = 126.4
Limiting Molar Conductivity
Whenever a solution is diluted up to infinity the molar conductivity of the solution at this point is known as limiting molar conductivity.
Furthermore, let us understand this topic with some example problems (1).
Problem: The following values represent the limiting molar conductivity of the compounds NaCl, HCl, and CH3COONa. Find the molar conductivity at infinite dilution of CH3COOH (Acetic Acid).
Λ∞ (NaCl) = 126.4 S.cm2.mol-1
Λ∞ (HCl) = 425.9 S.cm2.mol-1
Λ∞ (CH3COONa) = 91.0 S.cm2.mol-1
Solution:
We know that,
CH3COONa + HCl = CH3COOH + NaCl
On adding CH3COONa with HCl we get acetic acid and NaCl as byproducts.
So, in order to find the limiting molar conductivity of Acetic Acid we will simply add the limiting molar conductivity of CH3COONa and HCl and remove the limiting molar conductivity of NaCl.
Λ∞ (CH3COONa) + Λ∞ (HCl) – Λ∞ (NaCl) = Λ∞ (CH3COOH)
91.0 + 425.9 – 126.4 = 390.5 S.cm2.mol-1
Hence, the answer is 390.5 S.cm2.mol-1
Problem2: Calculate the molar conductivity (Λ0m) for CaCl2 , given:
Λ Ca2+ = 119.0 S cm2mol−1
Λ Cl⊖ = 76.3 S cm2mol−1
Solution:
Λ0m(CaCl2) = Λ Ca2+ + 2 ✕ Λ Cl⊖
(Since CaCl2 consists of one calcium and two chloride ions, so we add their molar conductivity to get the same of the compound)
Λ0m(CaCl2) = 119.0 + 2 ✕ (76.3)
( Substituting the values of molar conductivity of the respective ions.)
Λ0m(CaCl2) = 271.6 S cm2 mol-1 .
Problem 3: The molar conductivity of methanoic acid is 46.1 S cm2 mol-1. Calculate its degree of dissociation. Given λ0 (H+) = 349.6 S cm2 mol-1 and λ0(HCOO–) = 54.6 S cm2 mol-1 .
Solution
Degree of dissociation (α) = Λm/ Λ0m
(molar conductivity/limiting molar conductivity of the compound)
Λm of methanoic acid = 46.1 S cm2 mol-1 (Given in question)
λ0(HCOOH) = λ0 (H+) + λ0(HCOO–)
(Since formic acid or methanoic acid is formed by H+ ion and HCOO–, hence we will add molar conductance of them to get the same of the compound.)
λ0(HCOOH) = 349.6 + 54.6 S cm2 mol-1
λ0(HCOOH) = 404.2 S cm2 mol-1
Therefore,
α = 46.1/ 404.2 ( As discussed above)
α = 0.114
Hence, the answer is 0.114
Q&A
1. How to calculate the molar conductivity of a solution?
Λm = Κ * V
where V is a volume of solution,
K is conductivity.
2. What is molar conductivity?
Ans. The conducting ability of all ions present in a solution.
3. How to calculate molar conductivity?
Ans. Can be calculated by taking the product of the volume of the solution and its conductivity.
4. How to find molar conductivity?
By the formula:-
Λm = Κ * V
where V is a volume of solution,
K is conductivity.
5. Why does molar conductivity decrease with an increase in concentration?
In a solution, when we decrease the concentration, molar conductivity increases. It
happens because the volume of solution containing one-mole electrolyte, increases.
Also in the formula, we can see that,
Λm = Κ * V
When the volume of the electrolyte increases, molar conductivity increases.
