Know in one minute about glycosidic bond
|
Introduction
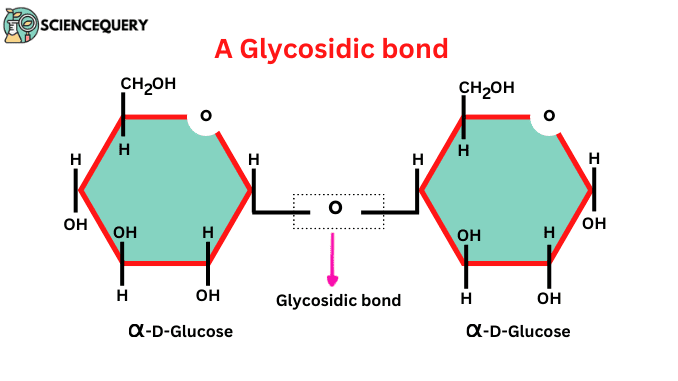
A glycosidic bond is a type of covalent bond that joins one carbohydrate (sugar) molecule to another molecule, which may or may not be a carbohydrate molecule. For example, Sucrose, cellulose, and maltose are formed by the joining of two or more monosaccharide units.
Definition
The type of covalent bond formed between the aldehyde or ketone group of one sugar molecule and the hydroxyl group of another sugar molecule is called a glycosidic bond.
- When two sugar molecules are joined by a glycosidic bond it forms a disaccharide
- Several sugar molecules are linked together and are known as oligosaccharides.
- And when a long chain of sugar molecules is linked it is called a polysaccharide.
Therefore, they are important for the formation of oligosaccharides and polysaccharides.
Structure
- It is formed between the hemiacetal or hemiketal group of saccharide and the hydroxyl group of some compounds such as alcohol.
- Monosaccharide includes a cyclic-hemiacetal structure in which there is an oxide ring between carboxyl carbon and C₄, C₅, or C₆ carbon atoms.
- The carbon in a cyclic sugar that is the carboxyl carbon in the open chain is called the anomeric carbon
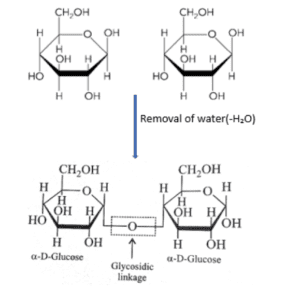
- During the formation, there is a removal of water molecules hence, this is a condensation process.
- Condensation takes place between a hydroxyl oxygen atom on carbon in one sugar and the anomeric carbon of another sugar.
When the alcohol group attacks the anomeric carbon, the OH group which is bound to that carbon is replaced by the oxygen of alcohol and it eliminates the hydrogen of alcohol. Therefore, there is the removal of OH and H in the form of water. These are quite stable but the addition of water molecules breaks.
Types of Glycosidic bonds
- Alpha
- Beta
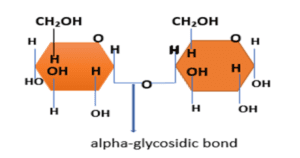
1. Alpha glycosidic bond
It is formed when the carbon of both the monosaccharides have the same stereochemistry. In this -OH group attached to C1 carbon below the plane of the ring, therefore the bond lies below the plane.
All the sugar molecules are in the same orientation.
Example- alpha 1,4- glycosidic bond
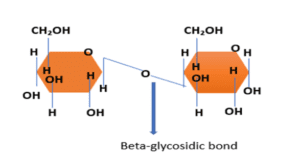
2. Beta glycosidic bond
This bond is formed when the carbon has different stereochemistry. The -OH group attached to C1 is positioned above the plane of the ring, and the bond lies above the plane. Adjacent sugar molecules are in the opposite direction.
Example- beta 1,4- glycosidic bond
3. Alpha 1,6- glycosidic bond
This bond is formed between the two or more identical alpha D – glucose molecules joined together. In this, condensation takes place between the hydroxyl oxygen on C-6 of one sugar and the alpha anomeric carbon of C-1 on the other.
Example- Amylopectin

4. Beta 1,6- glycosidic bond
It is formed when beta D – glucose molecule joins together. In this condensation takes place between the -OH group on C-6 of one sugar and the -OH group of C-1 on the other.
Example -Glucan
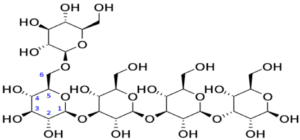
Importance of glycosidic bond
- It is a type of covalent bond that joins carbohydrate molecules to other or the same molecules it is important for the formation of oligosaccharides and polysaccharides.
- It forms the polymer unit of DNA by joining the 9th nitrogen of purine bases or the 1st nitrogen of pyrimidine bases linked with the 1st carbon of the sugar group.
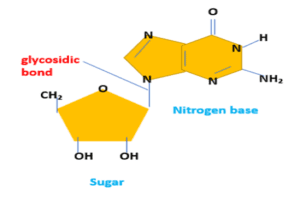
Glycosidic bond in DNA
It is present in DNA molecules between sugar and nitrogen bases to form the nucleoside. They is formed when the 9th nitrogen of purine bases or 1st nitrogen of pyrimidine bases is linked with the 1st carbon of the sugar group.
Beta vs Alpha glycosidic bonds
Beta glycosidic bond |
Alpha glycosidic bond |
| It is formed when the carbon has different stereochemistry. | It is formed when the carbon of both the monosaccharides have the same stereochemistry |
| The -OH group attached to C1 is positioned above the plane of the ring, and the bond lies above the plane. | In this -OH group attached to C1 carbon below the plane of the ring, |
| Bond lies above the plane | Bond lies below the plane |
| Adjacent sugar molecules are in the opposite direction. | All the sugar molecules are in the same orientation. |
Q&A
1. What are glycosidic bonds?
They are the bonds that are formed between the -OH groups of carbon atoms present in the monosaccharide by the removal of water molecules. It results in the formation of a long chain of sugar molecules.
2. What is glycosidic bond?
It is a bond that joins one carbohydrate (sugar) molecule to another molecule, which may or may not be a carbohydrate molecule. Formed between anomeric carbon and alkoxy oxygen of sugar molecules. During the formation, there is a removal of water molecules hence, this is a condensation process
3. Is glycosidic bond covalent?
Yes.
4. What type of glycosidic bond is present in maltose?
In maltose alpha 1,4- glycosidic bond is present that linked the two molecules of glucose. In this the -OH group of C-1 of one sugar is linked to the -OH group of C-4 of other sugar.
5. Which of the disaccharides has both anomeric carbon involved in the glycosidic bond?
In sucrose, two anomeric carbons are involved in the formation of glycosidic bonds.
6. Where is the glycosidic bond located in DNA?
In DNA they is located between the sugar molecule and the nitrogen base. The glycosidic bond is formed when the 9th nitrogen of purine bases or 1st nitrogen of pyrimidine bases is linked with the 1st carbon of the sugar group.
Reference
Shivalal Unified Chemistry Third/3rd/IIIrd Year – for MP Universities … Dr. S. C. AGARWAL
