.e
Know in one minute about Lactate dehydrogenase reaction
|
Introduction
The lactate dehydrogenase reaction is basically the anaerobic metabolism of glucose. That is when the oxygen is absent or is present in a very low amount then the pyruvic acid gets converted to lactic acid using NADH. It is a reversible reaction (1). This reaction is catalyzed by the lactate dehydrogenase enzyme (LDH). It plays an important role in producing the body’s energy.
Important points about Lactate dehydrogenase reaction
- It is an anaerobic reaction, which is a reaction that happens in absence of oxygen.
- This happens when the cell needs energy and uses the enzyme lactate dehydrogenase.
- The enzyme lactate dehydrogenase thus converts pyruvate to lactate.
Important points about Lactate dehydrogenase enzyme
- Lactate dehydrogenase is a cytoplasmic enzyme and an important enzyme of the anaerobic metabolic pathway and DNA metabolism.
- LDH enzyme is a type of oxidoreductase enzyme thus its reduced pyruvate to lactate and oxidized lactate to pyruvate. This is a reversible conversion.
- This enzyme is present in almost all tissues. But found in high concentration mainly in muscles.
- It is a marker of common injuries and diseases because it is released during tissue damage.
- Lactate dehydrogenase is the best example of an isoenzyme
- Two polypeptide chains, one is “H” and the second is “M”.
- The subunits composition of the LDH enzyme varies among tissues It is made up of five isomers.
There are five distinct enzymes, namely
Name |
No. of Subunits |
Tissue Source |
| LDH1 | H4 | Heart and RBC cells |
| LDH2 | MH3 | Reticuloendothelial cells and RBC cells |
| LDH3 | M2H2 | Lungs |
| LDH4 | M3H1 | Kidney |
| LDH5 | M4 | Liver and Skeletal muscles |
Definition
The lactate dehydrogenase reaction is the interconversion of pyruvic acid to lactic acid in the presence of the lactate dehydrogenase enzyme.
Lactate dehydrogenase is a hydrogen transfer enzyme that catalyzes the reaction by converting NADH to NAD+, It is a reversible reaction.
About Lactate dehydrogenase enzyme
- The lactate dehydrogenase enzyme serves as a key enzyme in the anaerobic metabolic pathway.
- LDH is responsible for the production of lactate and the regeneration of NAD. For example, when a person exercises hard, oxygen levels drop quickly, and muscles need to continue creating ATP, in this condition LDH converts pyruvate to lactate and generates NAD to produce energy. LDH in forming lactic acid removes electrons from NADH to complete the process.
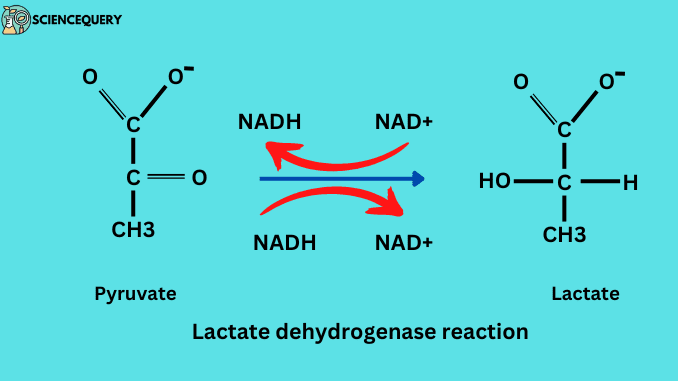
Lactate dehydrogenase reaction
The lactate dehydrogenase enzyme catalyzes the interconversion of pyruvic acid to lactic acid using NADH, that the same time catalyzes the same the catalyzes reverse reaction also. In this reaction, pyruvate was reduced to lactate, and lactate oxidized into pyruvate.
LDH catalyzes the reaction:-
Pyruvate + NADH → Lactate + NAD+
Lactate dehydrogenase mechanism
- It proceeds by transferring a hydride ion from NADH to pyruvate.
- Firstly NADH binds to the enzyme and many residues are involved in this binding.
- Hydride ion transfers in both directions and form two tertiary complexes, LDH- NAD+ + Lactate and LDH – NADH + pyruvate.
- Subsequently, pyruvate dissociated from the enzyme first, and NAD+ was released.
Examples of reactions catalyzed by lactate dehydrogenase
- LDH1 catalyzed inhibition of pyruvate, and then pyruvate converted into acetyl coenzyme and enters in TCA cycle.
- LDH5 enzyme does not inhibit pyruvate to lactate.
- In the Liver, LDH performs a reverse reaction of converting lactate to pyruvate through the Cori cycle.
- LDH catalyzes the dehydrogenation of 2-hydroxybutyrate
- LDH works as a catalyst in homolactic fermentation.
What is pyruvate lactate dehydrogenase?
Pyruvate lactate is an enzyme that converts pyruvate to lactate by reduction method. When the conversion of glucose into lactate meets a dead end in metabolism, lactate is released in the blood and transported to the liver, where LDH converts lactate to pyruvate.
Pyruvate undergoes oxidative decarboxylation to produce acetyl CoA. This reaction catalyzes by pyruvate dehydrogenase.
Carboxyl groups are removed with the release of carbon dioxide NAD+ is reduced to NADH and acetyl coenzyme is formed.
Acetyl coenzyme enters in TCA cycle to form carbon dioxide, water, and energy.
Q&A
What is the primary importance of the lactate dehydrogenase enzyme?
LDH important role in making energy, and maintaining homeostasis in the absence of oxygen. It is a marker of common injuries and diseases.
What is the lactate dehydrogenase reaction?
Lactate dehydrogenase catalyzed the reversible conversion of pyruvate to lactate using NADH, this reaction is called lactate dehydrogenase reaction.
Lactate dehydrogenase is what kind of reaction?
This is both an oxidation and reduction reaction also.
What is the reduction in a lactate dehydrogenase reaction?
In lactate dehydrogenase reaction pyruvic acid has been reduced NADH converts into NAD+
There are five isoenzymes of lactate dehydrogenase that catalyze exactly the same reaction.
LDH1, LDH2, LDH3, LDH4, and LDH5 are five isoenzymes of lactate dehydrogenase. LDH1 and LDH5 catalysts have the same reaction. LDH1 inhibited the conversion of pyruvate to lactate but LDH5 was not inhibited in pyruvate to lactate.
References
Written By: Richa Pachori
