
Introduction
Water, also called Hydrogen Oxide is formed by 88.89% oxygen and 11.11% hydrogen by weight. Water is a compound that possesses a definite composition by mass. There is evidence of chemical change in a compound formation in the formation of heat or light.
What is hydrogen oxide?
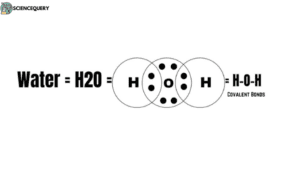
Hydrogen Oxide, also called H2O, is a strongly dipolar molecule. In water formation, two hydrogen atoms share two electrons with an oxygen atom. The remaining three atoms are in a stable state. The measure of the attraction of an atom for the shared electrons between it is called Electronegativity.
Why is water known as hydrogen oxide?
Water is known as Hydrogen Oxide because it is composed of two elements: Hydrogen and Oxygen. The empirical formula for water is H2O, which stands for two atoms of hydrogen (H) combined with one atom of oxygen (O). This combination results in the formation of a compound that is necessary to sustain life on Earth.
Chemical
Properties of Water
Molecular formula
The molecular formula for water is H2O.
Structure Of Water
- Water is made of two hydrogen atoms and one oxygen atom, thus forming a covalent bond. This structure provides water its unique properties like high surface tension, strong cohesive forces, and the ability as a universal solvent.
- The electronic configuration of the oxygen atom is 1s2 2s2 2p6.
- The molecular shape of water is bent because of the electronegativity between the hydrogen and oxygen atoms. It creates an asymmetric charge distribution in which both positive and negative charges are present. The oxygen atom has a partial negative charge while the hydrogens have partial positive charges. The intermolecular forces of attraction – hydrogen bonds give rise to some of water’s most important physical properties.
Physical Properties of Water
- Water is a clear and colorless liquid that is a vital element necessary to sustain the lives of animals and plants.
- The melting point of water is 0ºC or 32 ºF.
- The boiling point of water is 100ºC or 212ºF.
- The specific gravity of hydrogen oxide (water) is 4ºC.
- Water is an excellent solvent as it has the property to dissolve different substances.
- This solvent has a specific heat tolerance capacity that helps in regulating the marine environment. This property proves beneficial in the existence of the marine world.
- Marine life uses oxygen dissolved in water. Hence they use dissolved oxygen for respiration.
Chemical properties
- Water is amphoteric. Hence, it can react with both acids and bases in a neutralization reaction to form salt and water.
- Water is the only matter that is naturally abundant as a solid, liquid, and gas. A drop of water contains more than one billion water molecules.
- Water has a certain surface tension that helps in supporting the weight of insects, and other organisms.
- Water acts as a buffer as it resists changes in pH when any chemicals are added or removed from the solution. Thus it plays an important role in maintaining homeostasis in organisms.
- Due to extensive hydrogen bonding between molecules, the boiling point of water is much higher than other compounds.

Hydrogen bonding
It is the formation of bonds due to unequal electrons bonding between hydrogen atoms and oxygen. Hydrogen bonding plays a vital role in governing many physical properties like surface tension, dielectric constant, melting point, and boiling points.
Importance for living organisms
- A minute quantity of 0.0004% of the water on the earth’s surface is found in living organisms.
- Water is an important part of the plants. Plants carry out all kinds of processes such as the transportation of substances, photosynthesis, and maintaining the water pressure.
- From microscopic plankton, like algae to the largest animal – whales, water is the means of survival.
- Water is essential for human life as it makes up 70% of the body weight.
- Our body needs water to cope with the processes of digestion, respiration, and removal of toxic wastes from the body.
- Water is also needed for all agricultural purposes such as irrigation and to development of crop fields.
- It is also needed by the animals for drinking purposes.
- Water is extensively used in urban areas, and in factories to generate products and services useful for human beings.
- The water Blasting technique is used for shaping gemstones, and when sprayed at high pressure works like a knife.
- Every living organism needs water to quench its thirst and helps in maintaining osmoregulation.
Water cycle
Evaporation
Evaporation is the simple process of conversion of liquid into its vapor form. It occurs when molecules of a liquid break free and become steam. Water evaporates all the time from seas, lakes, rivers, and wet surfaces. This adds moisture to the air and finally falls as rain.
Condensation
Condensation is the process of a gas turning into liquid form. When the air becomes cool, its water vapor separates as droplets that form clouds. These clouds merge, become raindrops, and come back to earth.
Precipitation
It is a term to describe the different forms of water that fall or settle from the sky. It thus includes snow, sleet, hail, frost, and dew. This process shows how water in the air returns to the earth’s surface.
Percolation
It is defined as the movement of water through soil and rocks as groundwater. This water generally takes thousands of years to gather underground, also known as aquifers.
Transpiration
Transpiration is the evaporation of water from plants.
Technological applications
- Drinking: Water is essential for human life, as we need to drink clean and potable water every day to stay healthy.
- Irrigation: Water is needed in huge amounts to support agriculture and help the plants to grow better.
- Industrial: This important solvent is required in industries and factories for manufacturing products and generation of products. Water is also required in sanitization and cleaning processes in industries.
- Hydroelectricity: The generation of electricity using water is termed hydroelectricity. It is generated by the help of turbines that are driven by flowing water from dams or rivers.
- Transportation: Water transport of goods and trade relations are maintained well through inland waterways that enable the transport of goods.
Q&A
1. Does H2O mean hydrogen oxide?
Yes, technically H2O is the chemical formula for water. It comprises two atoms of hydrogen and one atom of oxygen.
2. Is H2O2 a hydrogen oxide?
No, H2O2 is called Hydrogen Peroxide.
3. What is H2O called?
H2O is called Water. Chemically, it is known as Hydrogen Oxide.
4. What is hydrogen oxide also known as?
Hydrogen Oxide is also termed water. It consists of two atoms of hydrogen and one atom of oxygen joined together by hydrogen bonds.
Summary
- Hydrogen Oxide, also called H2O, is a strongly dipolar molecule.
- In water formation, two hydrogen atoms share two electrons with an oxygen atom. The remaining three atoms are in a stable state.
- The measure of the attraction of an atom for the shared electrons between it is called Electronegativity.
- Water is a compound that possesses a definite composition by mass.
- hydrogen atoms and oxygen form bong in water.
- This bonding plays a vital role in governing many physical properties like surface tension, dielectric constant, melting point, and boiling points.
- Water is the only matter that is naturally abundant as a solid, liquid, and gas.\
- Water is essential for human life as it makes up 70% of the body weight.
- Our body needs water to cope with the processes of digestion, respiration, and removal of toxic wastes from the body.
- Water is also needed for all agricultural purposes such as irrigation and to development of crop fields.
- It is also needed by the animals for drinking purposes.
- The generation of electricity using water is termed hydroelectricity. It is generated by the help of turbines that are driven by flowing water from dams or rivers.
- Water is extensively used in urban areas, and in factories to generate products and services useful for human beings.
References
- https://books.google.com/books?hl=en&lr=&id=dr-VBAAAQBAJ&oi=fnd&pg=PP1&dq=water+chemistry&ots=0cL_GD0-LC&sig=Ir2EIWfaaTRWWk-WOEbmPmT_-ws
- https://iopscience.iop.org/article/10.1088/0953-8984/21/28/283101/meta
- https://www.annualreviews.org/doi/pdf/10.1146/annurev.bb.03.060174.000523
- https://www.researchgate.net/profile/T-Harrold/publication/260072736_The_global_water_cycle/links/5f1798f4299bf1720d58d0eb/The-global-water-cycle.pdf
- https://pubs.rsc.org/en/content/articlehtml/2021/sc/d0sc06000c
- https://books.google.com/books?hl=en&lr=&id=h0mvDwAAQBAJ&oi=fnd&pg=PR5&dq=water+chemistry&ots=YIlBRH7DVB&sig=h0J9OaCwr2jKe9MmYoXmVDuCNoU
Written By: Sushmita Mukhopadhyay
