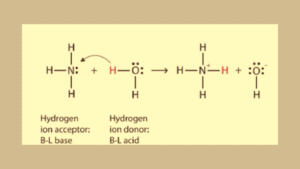
Introduction
In 1923 Bronsted and Lowry proposed this theory to define acid and base. It’s applied on both aqueous and non-aqueous solution. They focused on the movement of hydrogen ions.
Definition
According to this theory, Acids are substances (molecules or ions) that have the tendency to donate protons (H+) and bases are substances that are acceptors in nature meaning they accept protons in a reaction.
Comparison with other acid-base theory
- According to Arrhenius’s theory acids are substances that give H+ ions in a solution and base gives OH- in a solution. This theory is not capable of defining all substances.
- Bronsted Lowry’s theory says compounds that can donate Hydrogen ions are called acids and compounds that accept a hydrogen ion are called bases.
- This theory is useful for its ability to handle any protonic solvent like liquid ammonia or sulfuric acid.
Bronsted –Lowry acid
Properties
Proton donating nature
- Bronsted–Lowry acid dissociates and provides H+ or proton. They are also called protogenic.
- Explanation of acid dissociation and proton donation
- In acid base reaction acids are dissociated in positive ions and negative ions. Protons are positive ions.
- Protons are electron-deficient species and the base is capable of giving lone pairs of electrons. Hence proton is moved to base from acid which is called proton donation.
Bronsted- Lowry base
Properties
Proton accepting nature
- Bronsted-Lowry base accepts protons from acid. They are also known as protophilic.
- Base acceptance and protonation
- A Bronsted Lowry base is a substance that is capable of accepting a proton from losing a lone pair of electrons. For completing valence shell duplet proton bind with base it’s called ptonation. Base is an electron-rich species hence base gives electrons for binding with protons.
Acid-base equilibrium
- Acid-base equilibrium means there is a balance between two opposing processes and no change occurs.
- This chemical reaction is represented with a double reversible arrow. It’s applied to weak acids and bases.
- There are both forward and reverse reactions occurring, and their effects cancel each other out. This process is called chemical equilibrium and it balances out with each other.
- When a chemical reaction reaches equilibrium, the reaction stops because the reverse reaction balances out the forward reaction.
- For example, the ionization of the weak acid HC2H3O2 (aq) is as follows:
Acetic acid dissociates and donates an H+ to water molecules and forms H3O+ and C2H3O2− .
HC2H3O2 (aq) + H2O (l) → H3O+ (aq) + C2H3O2−(aq)
The reverse process also begins to occur:
In the reverse reaction H3O+ and C2H3O2− ions again formed acetic acid and water molecules.
H3O+(aq) + C2H3O2− (aq) → HC2H3O2(aq) + H2O
Examples of the reaction which involves Bronsted-Lowry acid-base
This reaction demonstrates the Bronsted-Lowry definitions of an acid and a base. Ammonia reacts with water and produces ammonium ion and hydroxide ions (OH–) as a product.
In this reaction water molecule donates a hydrogen ion to the ammonia so its Bronsted Lowry acid while the ammonia accepts the hydrogen ion so it is the Bronsted base.
Reaction of nitric acid with water
Nitric acid acts as Bronsted-Lowry acid because it donates H+ to the water molecule. Water acts as a Bronsted-Lowry base because it accepts hydrogen ions.
HNO3 + H2O → H3O+ + NO3–
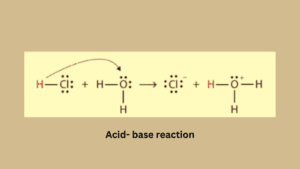
Acid-base reaction
The acid-base reaction is based on the transfer of H+ ions from an acid to a base. Firstly acid like HCL dissociates in proton (H+) and chloride ion (Cl–).
Protons are electron-deficient species because they lost an electron which is present in their valence shell. It is an unstable form of proton for coming stable form protons need a lone pair of electrons.
The resulting proton binds with the base to complete its valence shell while ammonia is an electron-rich species that’s why it easily accepts protons.
Example
HCL + NH3 → NH4+ (Conjugate acid) + Cl– (conjugate base)
Conjugate acid– A base after accepting a proton becomes an acid and is known as conjugate acid.
There is a difference of one proton between the base and its conjugate acid. If the base is strong its conjugate acid will be a weak acid.
Conjugate base– An acid donating a proton becomes a base and is known as a conjugate base. If acid is strong its conjugate acid will be weak.
Acidic and basic strength
- Acids and bases do not all demonstrate the same degree of chemical activity in solution. Different acids and bases have different strengths.
- An acid that is 100% ionized in an aqueous solution is called a strong acid and an acid that is less than 100% ionized in an aqueous solution.
- HCl (aq) is an example of a strong acid because it is 100% ionized in H+ ions and OH– ions. Acetic acid is an example of a weak acid.
- A base that is 100% ionized in an aqueous solution is called a strong base and a base that is less than 100% ionized in an aqueous solution is called a weak base.
- Food products that are contained in weak acid solutions become slightly acidic. If they contain strong acids the food would likely be inedible.
There are some examples of strong acids and bases
| Strong acid | Strong base |
| HCl | LiOH |
| HBr | NaOH |
| HI | KOH |
| HNO3 | Mg(OH)2 |
| H2SO4 | Ca(OH)2 |
Amphoteric species with examples
- A substance that can either donate or accept a proton, depending on the circumstances, is called an amphoteric compound.
- Water is an amphoteric species that acts as both Bronsted Lowry base and Bronsted Lowry acid.
- Water is not only one of the examples of amphoteric species. Aluminum hydroxide [Al (OH)3] is also amphoteric in nature. It reacts with both acids and bases.
Examples
1. NH3 + H2O → NH4 ++ OH–
In this acid-base reaction water (H2O)
acts as an acid and NH3 acts as a base.
2. HNO3 + H2O → H3O+ +NO3–
In this reaction, water acts as base and HNO3 acts as acid.
Q&A
1. Bronsted Lowry acid and base definition?
Bronsted Lowry acids are proton acceptors which means they give a proton and Bronsted Lowry bases are proton acceptors which means they accept protons.
2. Bronsted Lowry acid and base theory?
Bronsted Lowry’s acid and base theory is based on the movement of protons. According to this, acids are proton donor and base is proton acceptor.
3. Bronsted Lowry acid and base reaction?
The acid-base reaction is based on the transfer of H+ ions from an acid to a base.
4. According to Bronsted and Lowry, what is a base?
According to Bronsted and Lowry, a base is a substance that accepts a hydrogen molecule from acids.
Summary
- According to Bronsted-lowry acids are a substance that gives protons and base is a substance that accepts protons.
- Acid and base react and acid transfers protons from the base. Acids form conjugate bases and base forms conjugate acid.
- Acids and bases can be strong or weak depending on the extent of ionization in the solution.
- Most chemical reactions reach equilibrium at which point there is no net change.
- In a reaction, acids dissociate in positive and negative ions. Protons are positive ions that form from loose her lone pair of electrons.
- It is an unstable form for a stable form; a proton needs a lone pair of electrons. Which is given by base hence proton binds with base.
- A base accepting a proton is converting to conjugate acid and an acid donating a proton is converting to conjugate base.
- If the base is strong its conjugate acid will be weak acid and if the acid is strong its conjugate acid will be weak.
- Water molecule belongs to the amphoteric species because it’s used as both acid and base.
- An acid or base that is 100% ionized is called a strong acid or base. If they are less than 100% ionized it is called weak acid or base.
- When a chemical acid-base reaction reaches equilibrium, the reaction stops because the reverse reaction balances out the forward reaction.
References
- Introduction to Chemistry (General, Organic, and Biological v. 1.0)
- Concise inorganic chemistry, 4th edition, J.D. Lee
- Inorganic Chemistry (Principal of structure and reactivity), 4th edition, James E. Huheey, Ellen A. Keiter, Richard L. Keiter