Know in one minute about tautomerization
|
Introduction
Tautomerism is a type of isomerism. It’s also known as desmotropism and dynamic isomerism. Where they have two different structures of the same molecular formula. Due to the oscillation of a monovalent (hydrogen) atom between polyvalent atoms. Which compound has one & more valiancy is called polyvalent. Oscillation means, the to and fro motion of hydrogen. Hydrogen moves from one atom to another atom, it changes the structure of the compound. Hydrogen changes its position in between two polyvalent atoms. Its movement or phenomenon is called 1, 3 hydrogen migration.
Definition
The process of shifting a hydrogen atom from one carbon atom to another to produce tautomers is known as tautomerization.
Other Names
Other names of tautomerism are kryptomerism, allelo-tropism, or merotropy, however, tautomerism is the commonly used term.
Occurrence
Tautomerism can occur in planar as well as non-planar molecules.
Tautomerization takes place in the presence of an acid-base catalyst
In the acid-catalyzed reaction, the molecule is first protonated on oxygen it’s called protonation, and then loses a proton from C in a second step, it is called deprotonation.
Base-catalyzed reaction, the C–H proton is removed first by the base, a hydroxide ion, and the proton is added to the oxygen atom in a second step.
In an acid-catalyzed reaction, we get protons again, and at the end of a base-catalyzed reaction, we get hydroxide ions back again.
Tautomers
Definition and types
Tautomers are isomers of a compound, which differ only in the position of the protons and electrons. The carbon skeleton of the compound is unchanged but functional groups are different. The – hydrogen of carbonyl compounds is acidic, as it is connected with the carbon that is directly bound to the electron-withdrawing carbonyl group. This is the main reason for tautomerism to occur. The acidities of these α-hydrogen atoms are enhanced if an electron-withdrawing group is attached to the α-carbon atom.
The establishment of equilibrium may be catalyzed by both acids and bases. In the absence of any acid and any base, the Keto and the enolic form are separated from each other by fractional crystallization or distillation.
Type of tautomerism
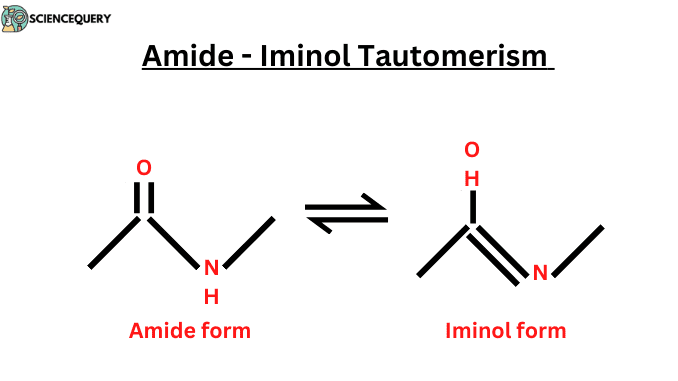
- Amide-Iminol (amide-imidic acid) tautomerism
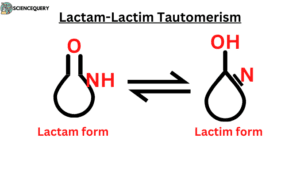
- Lactam – Lactim tautomerism
- Amine-Imine tautomerism
- Annular tautomerism
- Valence tautomerism
- Non-prototropic tautomerism
- Ring-chain tautomerism
- Factors influencing Tautomerization
Keto-enol tautomeric form
- Keto-enol is one of the most important examples of tautomerism.
- The enol must be formed by a transfer of a proton from the central CH2 group of the Keto form to one of the OH groups, a reaction is known as enolization.
- There is no change in pH—a proton is lost from carbon and gained from oxygen. The only change is the transfer of one proton and the shift of the double bond.
- This type of Interconversion is called tautomerism and these compounds are called tautomers.
- Generally, Keto tautomer is more stable than enol groups. Keto and enol are functional groups. Enol has a hydroxyl group (-OH) attached to alkenes (C=C).
Example of Keto-enol tautomeric form
- acetone and phenol
- acetoacetic ester
Mechanism
1. Proton transfer mechanisms
- The process of tautomerization or enolisation can be catalyzed by either acid or base. Under both these conditions the acidity of the alpha-hydrogen is the main influence on this change.
- That is, the action of enolisation is due to the acidic nature of the alpha hydrogen atom. In carbonyl compounds the acidity of alpha hydrogen arises due to two reasons.
- One of the reasons is the electrophilic nature of the carbonyl group and the second reason is, the stability of an anion or enolate ion formed when a proton is ejected from the alpha position, which becomes more stable by resonance.
- The enolate ion is negatively charged so the electron density is more on the alpha carbon. In addition, protons on the oxygen of the enolate ion are formed. In this also electron density is more on the alpha carbon.
2. Rearrangement mechanisms
Rearrangement mechanisms mean one atom or group moves one carbon atom to another. This reaction also includes the breaking and making of bonds between two carbon atoms.
Acidic Medium
These are the following steps in an Acidic medium
- H+ ion is present in an acidic medium, which needs electrons in this condition.
- Oxygen reacts with hydrogen and donates its lone pair electron which gives a positive charge to oxygen.
- The electronegativity of the oxygen is already more and the positive charge will come and a double bond will be pulled toward itself, so carbocation will form.
- CH3 will donate hydrogen to stabilize the carbocation.
- In step 1 bond formation takes place, in the second step resonance takes place then the sigma bond breaks, and a new bond is formed.
Basic medium
These are the following steps in Basic medium
- OH– ion is present in the basic medium; the nature of α-hydrogen is acidic because the nature of the carbonyl group is electron withdrawing.
- Hydrogen ions were removed from α carbon and formed alkene pi bonds. Then OH binds with Hydrogen and resonance takes place.
- Negatively charged oxygen reacts with HOH and forms enol.
Factors influencing Tautomerization
1. Solvent effects
If the solvent is polar it increases the percent of keto tautomers. Polar solvent, which is capable of forming hydrogen bonding, will make lone pairs less available for intramolecular H-bonding, hence % of keto increases.
2. Temperature and pressure
Temperature affects tautomerism. High-temperature range reduces their hydrations and makes them unstable thereby they dissociate into smaller aggregates.
- Substituent’s effects
- Steric hindrance
Applications
- Chemical structure of compound:- Tautomerism is useful for determining the chemical structure of compounds.
- Importance in nucleic acid: The presence of multiple tautomers increases the structural and chemical diversity of nucleic acid bases.
- Tautomerization of phosphoenolpyruvate: Phosphoenol pyruvate is a high-energy compound whose breakdown is essential for the generation of ATP. Firstly it’s hydrolyzed to pyruvate. Pyruvate enol form converts to Keto form. This breakdown is responsible for ADP to ATP formation.
Q&A
1. What is tautomerization with an example?
The process of transferring a hydrogen atom from α-Carbon to another to produce enediols is known as tautomerization.
2. What is the difference between resonance and tautomerization?
Tautomerism involves a change in the position of an atom while resonance involves a change in the position of the unshared or π electron only. Tautomers are definite compounds and may be separated and isolated. Resonating structures are only imaginary and can’t be isolated.
3. What is tautomerization under basic conditions?
Under Basic conditions, deprotonation occurs in an alpha hydrogen atom and the second step proton added to the oxygen it’s called protonation.
4. What causes tautomerization in DNA?
In DNA, tautomerization causes mutation. The formation of an alternative tautomer is a frequent source of errors during DNA synthesis.
References
- Organic chemistry, 2nd edition, Jonathan Clayden, Nick Greeves, and Stuart Warren
- Biochemistry By Satyanarayana & U. Chakrapani, 5th edition.
