Introduction
There are currently 118 elements in nature. These elements are arranged according to their atomic mass by Russian scientist Mendeleev and German scientist Lothar Meyer. This is known as Mendeleev’s periodic law. The elements that are in group 9 according to Mendeleev’s periodic table and in group 18 according to the modern IUPAC long periodic table, are inert elements or a noble gas.
In 1921 the scientist Moseley experiment showed that not the atomic masses, atomic numbers are the fundamental properties of the element. That is, the atomic number or the number of protons in the nucleus of the atom determines the chemical properties and nature of the element.
For this Mendeleev’s periodic law was later revised. The periodic table is the list of elements that are arranged according to the increasing atomic number according to the periodic law. According to Mendeleev’s revised periodic table, there are 7 periods and nine groups in the periodic table. And according to the modern IUPAC long periodic table, there are 7 periods and 18 groups in the periodic table (1) & (3).
What is a noble gas?
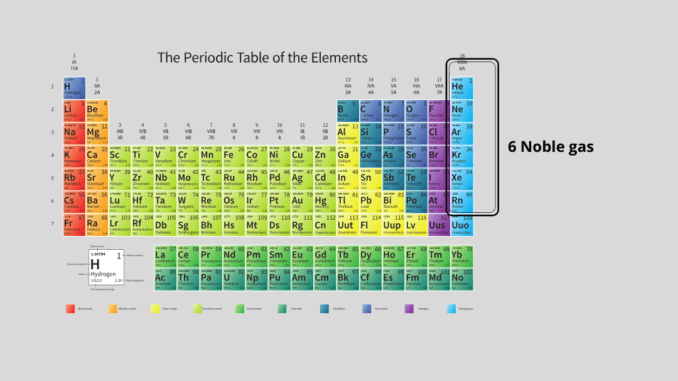
Nonmetals are located to the right of the periodic table. Of these nonmetals, there are 6 elements that do not normally participate in any chemical reaction. These are called inert elements or noble gases. These are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). Without radon, five other noble gas is present in very small amounts in the air. That is why noble gas is called rare gas (1) & (2).
Description of noble gas
Name |
Atomic number |
Discoverer |
Discovery year |
ElectronPer shell |
| 1. Helium | 2 | Janssen, Lockyer, and Cleve ( after Ramsay) | 1868 | 2 |
| 2. Neon | 10 | Ramsay & Travers | 1898 | 2, 8 |
| 3. Argon | 18 | Lord Rayleigh & Ramsay | 1894 | 2, 8, 8 |
| 4. Krypton | 36 | Ramsay & Travers | 1898 | 2, 8, 18, 8 |
| 5. Xenon | 54 | Ramsay & Travers | 1898 | 2, 8, 18, 18, 8 |
| 6. Radon | 86 | Dorn | 1900 | 2, 8, 18, 32, 18, 8 |
Reason for naming the noble gas or inert gas
Noble gas is monoatomic. They are chemically inert as their outermost orbit or valence orbit is completely filled with an electron. That is, elements do not usually react with any other elements to form compounds.
So their valence is zero. And they are chemically inert. So these 6 elements (helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) are called inert gas or noble gas (3).
Position of noble gas in the periodic table
Helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – these 6 noble gases are located in group zero or nine according to Mendeleev’s periodic table. The existence of these noble gases was not known when Mendeleev published the periodic table.
However, after the discovery of the elements, it was found that atoms of noble gases other than helium have eight electrons in the outermost cell. In the case of helium, there are two electrons on the outer cell.
The electron configuration of noble gases is very permanent in nature. So there are many similarities in their chemical properties. That is why Ramsay arranged for these six identical elements to be placed in a separate group in Mendeleev’s periodic table. He called this group the zero group (1) & (2).
Mendeleev’s periodic table (modern version)
However, the modern periodic table has seven periods and eighteen groups. As a modern long-form periodic table, periods are marked by numbers from one to seven as before, and groups are numbered 1 to 18 consecutively to the left of the table. According to the modern IUPAC long periodic table, the noble gases are located in groups 18 (1) & (2).
Amount of noble gas present in the atmosphere
Helium, neon, argon, krypton, xenon, and radon of the 6 noble gases, except for radon, all noble gases are present in very small amounts in the atmosphere. The amount of noble gases in the atmosphere is given below.
| Noble/inert gas | The amount of presence in the atmosphere (%) |
| Helium (He) | 0.0005 |
| Neon (Ne) | 0.0015 |
| Argon (Ar) | 0.9320 |
| Krypton (Kr) | 0.0001 |
| Xenon (Xe) | 0.00001 |
| Radon (Rn) | – |
Radon gas is the heaviest of the inert gases or noble gases, so it is not present in the atmosphere. Among the noble gases, the presence of argon is the highest in the atmosphere and the presence of xenon is the least (1).
Properties of noble gas
- Noble gases do not normally participate in any chemical reaction.
- Except for radon, five other inert or noble gases are present in various small amounts in the atmosphere.
- Noble gases have very low boiling and melting points.
- Of the six inert or noble gases, the first five elements are table.
- Inert or noble gases are colorless and odorless.
- The valence of inert or noble gas is zero.
- The inert or noble gases are gaseous and monoatomic.
- Inert or noble gases are slightly soluble in water. Solubility increases as the atomic mass increases.
- Except for helium, the other five inert or noble gases have eight electrons in their last cell (2).
Uses of noble gas
Helium
- Aircraft and gas balloons use helium due to its lightweight.
- Deepwater divers use a mixture of oxygen and helium to breathe.
- NMR machines use liquid helium for cooling
- A mixture of oxygen and helium is used for the breathing of asthma patients.
Neon
- This gas is used in lamps and colorful lighting
- Neon-helium mixture is used to store voltmeters and rectifiers.
Argon
- Electric bulbs sometimes use argon instead of nitrogen.
- The radioactive measuring instrument Geiger Muller counter has argon gas.
Krypton
Tube lights and electric gas bulbs use Krypton gas
Xenon
Xenon gas is used in photography flashlights.
Radon
It is used in cancer treatment and radiotherapy (1) & (2).
How alkali metals are different from noble gases?
Lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) are located in group I A of the Mendeleev’s periodic table and group 1 in the long-form periodic table, are called alkali metals.
These metals react with water at normal temperatures to produce alkali. That is why these metals are called alkali metals. These metals (alkali metals) in the first group of the periodic table are completely different from the noble gases located in the last group of the periodic table. Because-
- First of all noble gases are nonmetal. However, alkali metals are metal, which means the properties of metals are present in alkali metals.
- Noble gases are colorless. Alkali metals are colored (e.g., potassium (K) is silvery-white in color)
- Noble gases are gaseous under normal conditions. However, alkali metals are solid under normal conditions, except cesium and francium. They are liquid under normal conditions.
- Noble gases are inert or inactive. That is noble gas does not form compounds by reacting with any elements at normal temperature. However, there are exceptions. There are some noble gases that do not react to normal temperatures but react to extreme temperatures. For example, xenon gas reacts with F and O to form fluoride and oxide compounds.
Xe + F₂ → XeF₂ (Xenon difluoride)
On the other hand, alkali metals are very reactive metals. Alkali metals form compounds by reacting with water at normal temperatures. For example, sodium reacts with water to form sodium hydroxide.
2Na + 2H₂O → 2NaOH (sodium hydroxide) + H₂
- The valence of noble gas is zero. Because their outermost shell is full of electrons. They have eight electrons in their outermost shell (except helium, which has two electrons in its outermost shell). That is why noble gases are stable. So the tendency of noble gas to lose or gain electrons is zero. So this noble gas valence is zero.
On the other side, the alkali metals valence is one. This is because these metals have one electron in the outermost shell. They want to lose one electron at a time and become stable. So they have one electron in each of the outermost shells. That is why their valance is one.
The figure above describes the electron configuration of sodium (alkali metal) and argon (noble gas). It turns out that sodium has one electron in the outermost shell, so the valance of sodium is one. And argon has eight electrons in the outermost shell. So argon is stable. That is why argon valance is zero.
Conclusion
According to the modern IUPAC long-form periodic table, the noble gases are located in group 18. According to Mendeleev’s periodic table, these elements are located in group zero. Inert or noble gases are sometimes called the helium family or the neon family. Noble gases are very important. The discovery of inert or noble gas plays an important role in theoretical chemistry.
