Nernst equation: Definition and description

How to define the Nernst equation
This equation was named after German scientist Walther Nernst. It is important in electrochemistry as it provides a relation between the cell potential of an electrochemical cell.
Now the next question is what is the cell potential of an electrochemical cell?
Well, the cell potential of an electrochemical cell is the difference between its cathode and anode or it is the measure of the potential difference between two half cells in an electrochemical cell.
Let us simplify the term cell potential
Electrochemical cells are made up of two half-cells. Now each half-cell is made up of metal electrodes that are submerged in the same metal solution. And these half-cells are connected with each other with the help of a salt bridge.
The metal electrode of one of the half cells acts as an anode (Oxidation occurs and thus are positive terminal) and another one acts as a cathode (reduction occurs and thus are negative terminal).
The anode is positively charged due to oxidation as a result more and more electrons are released into the solution. Whereas cathodes are negatively charged due to reduction thus electrons are absorbed from the solution.
The function of the salt bridge in the electrochemical cells
Now due to anode and cathode oxidation and reduction reactions happen (Jointly called redox reaction) This oxidation results in the release of electrons into the solution and reduction does the opposite. to maintain a balance between these two half-electrochemical cells a salt bridge is inserted. It absorbs excess electrons and also releases electrons where there is scarcity. Thus this salt bridge is responsible for the flow of electrons thus it is for the flow of energy.
What is the relationship between the electrochemical cell and cell potential?
This cell potential is a type of energy that drive the movement of electrons from abundance to deficiency. The electrons of the anode are on higher potential than the cathode and thus drive the redox reaction and electron flows which ultimately are responsible for what cells are used to give the power to operate something.
Hence cell potential is
E0cell = Eredu, cathode– Eredu, anode
Where E0cell is standard cell potential (Under 1M, 1 Barr, and 298 K)
Eredu, cathode = Is the reduction reaction in the cathode
Eredu, anode = Oxidation reaction in the anode
Gibbs free energy
Gibbs free energy is a quantity that is used to measure the maximum work done in a thermodynamic system when the temperatures and the pressure are kept constant. It is denoted by the symbol “G”. Gibbs free energy is a simple formula showing a relation between enthalpy and entropy and temperature.
Enthalpy: It is denoted by (H): It is the sum of internal energy, pressure, time, and volume. It cannot be measured but we can calculate the change in enthalpy that is ΔH.
Entropy: It is the measurement of the degree of randomness and is denoted by S.
Therefore, the Gibbs free energy is G = H – TS
G = U + PV – TS
where G = Gibbs free energy
H = Enthalpy
S = Entropy
T = Temperature
U = Internal energy
P = Pressure
V = Volume
Note: The delta G sign is always negative for spontaneous reactions and is positive for non-spontaneous reactions.
Gibbs energy in electrochemistry is
ΔGo = -nFEo (Standard condition)
Electric work
The energy derives all the changes including the chemical reactions. In a redox reaction, the energy released in a reaction due to the movement of charged particles gives rise to a potential difference. The maximum potential difference is known as the electromotive force (EMF). And E is the maximum electric work
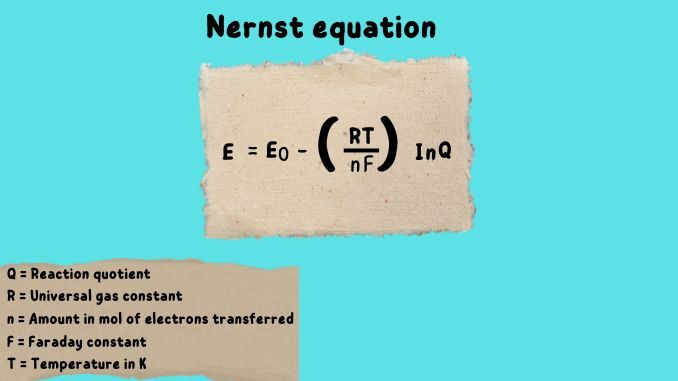
Nernst Equation
In electrochemistry, the Nernst equation is the most important. The Nernst equation provides a relation between the cell potential of an electrochemical cell (That is the potential difference occurring between the two electrodes of the cell that is the difference between cathode and anode).
Nernst equation
Ecell = Eo– RT/nF ln (Q)
Where Ecell= Cell potential in volts (Joules per coulomb)
n = moles of electrons
F = Faradays constant that is 96,485 coulombs per mole of electrons
Since, ln(x) = 2.303 log (x)
Therefore, Ecell = Eo -2.303 RT/nF log (Q)
Standard temperature T = 298 K
hence 2.303 RT/nF becomes 2.303X R X298/96458 = 0.0592. (1)
Therefore, Ecell = Eo -0.0592/n log (Q)
Derivation of Nernst equation from Gibbs free energy under standard condition
E0cell = Eredu, cathode– Eredu, anode
ΔG = -nFE (general condition) (2)
Where n= number of electrons transferred in a reaction
F = Faraday constant
E = Potential difference
ΔGo = -nFEo (Standard condition) (3)
Gibbs’ free energy equation is
ΔG = ΔGo + RT ln Q
putting the value from equations (2) and (3)
-nFE = -nFEo + RT ln Q (By canceling -nF from both sides we get)
E = Eo – RT ln Q
E = Eo – 2.303 RT log10 Q (In the form of Log10)
Under standard conditions from equation (1) we get
E = Eo – 0.0592V/n log10 Q
Applications of Nernst equation
This equation is used in the calculation of solution pH, constant equilibrium solubility product, to determine the cell potential under nonstandard conditions, etc.
This equation is also used in the food industry, fiber industry, extraction of essential oil, flavoring, etc.
removal of phenol from the waste eater.
Also used for the determination of association
And for the determination of stability.
Applied to full-cell reactions or half-cell reactions.
Use to calculate the electrode potential of a cell under general conditions.
Limitation of Nernst equation
To determine the Nernst equation following conditions should be taken care
- The temperature should be kept constant throughout the experiment.
- The molecular state of the solute has to be the same in the two solvents.
- The concentration of the solute is required to be noted after the equilibrium has been established.
Q&A
1. What does the Goldman-Hodgkin-Katz equation take into account that the Nernst equation does not?
The Nernst equation describes the relationship between the reduction potential and the standard electrode potential. but the Goldman equation describes the reversal potential across the cell membrane.
2. What is Nernst equation?
Nernst equation is
Ecell = Eo – 0.0592/n log (Q)
where, E = Cell potential
Eo = Standard potential
n = number of electrons
3. When to use Nernst equation?
Nernst equation is used to determine the cell potential.
