Enzymes: An introduction
Enzymes are biological catalysts that help in speeding up a chemical reaction without altering itself. All the enzymes are protein in nature except some small groups of RNA molecules called ribozymes. They are produced by the living cells of plants and animals. All the enzymes are colloidal in nature that is they are not soluble but stay suspended in a solution. They are also thermolabile because the enzymes lose their activity when the temperature rises. Basically, they are the biochemical catalysts and the catalysis or the process is known as biochemical catalysis or enzyme activity.
The biological process that occurs within all living organisms are chemical reactions therefore most of the processes are regulated by enzymes. Before discussing the enzymes types and their effect on chemical reactions in living cells, we should know some of the important things about enzymes
1. Substance
A substance is a matter which has specific nature and specific properties. Every compound and element can be a substance.
For example, iron is an element hence it is a substance. Methane is a compound therefore it is also a substance.
2. Catalyst
A catalyst is a substance that is an element or a compound that increases the rate of chemical reactions without altering itself.
For example, chlorine acts as a catalyst.
The function of enzymes in living cells
Enzymes are proteins that help to speed up chemical reactions in our bodies. Enzymes catalyze all aspects of cell metabolism. These are essential for digestion, liver function, healthy growth, and blood coagulation. Each cell in our body contains DNA. Each time a cell divides, the DNA thus needs to be copied. Enzymes help in this process by unwinding the DNA coils and copying the information. The liver breaks down toxins in the body, for this, it uses a range of enzymes. The enzymes in our body also help to perform a very important task in building muscles and protein mass.
The industrial function of enzymes
Enzymes also have valuable industrial and medicinal applications. For example, curdling of cheese, laving of bread, fermenting of wine, etc. The uses of enzymes in medicine include inhibiting disease-causing microorganisms. therefore, enzymes are valuable in our daily lives.
Nomenclature of enzymes
The naming of enzymes can be done according to the substance on which they act.
-
By adding the suffix -ase
Naming is done by adding the suffix -ase to the name of the substrate.
For example, Sucrase – Catalyzes the hydrolysis of sucrose
Lipase – catalyzes the hydrolysis of lipids.
-
Type of reaction with a suffix -ase
Naming is also done according to the reactions they catalyze by adding the suffix -ase to the kind of reactions they are catalyzing.
For example, Dehydrogenase – dehydrogenation reaction (removal of hydrogen).
Hydrolase – Hydrolysis of the substrate (breaking the bong using water).
Such a type of naming is also known as systematic naming proposed by the international union of Biochemistry.
Types of enzymes
According to the International Union of Biochemistry (IUB), enzymes are divided into six functional classes. These six functional classes are
- Oxidoreductase
- Lyases
- Transferase
- Ligases
- Isomerases
1. Oxidoreductase
Oxidation is the loss of electrons during a reaction by a molecule, atom, or ion. When the oxidation state of a molecule, atom, or ion is increased then oxidation occurs. the opposite of this reaction is called reduction which means the gain of electrons during a reaction. This catalyzes the removal or addition of hydrogen atoms, oxygen atoms, or electrons from one substrate to another, therefore, they are also called oxidases, reductases, or dehydrogenases.
Example: Cytochrome oxidase, alcohol dehydrogenase.
Alcohol + NAD ⇔ Acetaldehyde + NADH2
Alcohol dehydrogenase
2. Lyases
Catalyze the breaking of various chemical bonds without using water molecules.
Example: Decarboxylases → Removal of carboxyl (RCOOH) group from CO2. and also decarboxylases remove carbon dioxide from amino acids and dehydrases remove water.
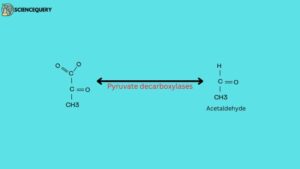
Other examples of lyases are phenylalanine ammonia lyase, citrate lyase, isocitrate lyase, etc.
3. Transferase
Transferase is another kind of enzyme that catalyze the transfer of specific functional groups from one molecule to another functional group means a group of atoms responsible for the characteristic reaction of particular compound examples of functional groups are
Hydroxyl, ketone, amine, methyl, carbonyl, carboxyl, etc.
Example
Hexose + ATP → Hexose-6-phosphate + ADP
(Hexokinase)
- Transaminase catalyzes the transfer of the amino group (-NH2) of an amino acid to a carbonyl compound.
- Transmethylase catalyzes the transfer of a methyl group.
- Phosphorylase catalyzes the addition of a phosphate group.
4. Ligases
A ligase in an enzyme can catalyze the joining of two molecules by forming a new chemical bond in the presence of ATP.
Example
Actyl CoA + CO2 + ATP → Maionyl-CoA + ADP + Phosphat
(Acetyl CoA carboxylase)
Other examples are 1. Succinate thiocyanate
2. Glutamine synthetase
3. DNA Ligase
5. Isomerases
This enzyme catalyzes reactions involving a structural rearrangement of a molecule. Isomerases catalyze changes within one molecule. They convert one isomer to another, meaning that the end product has the same molecular formula but a different physical structure.
Example
Trios phosphate isomerase, phosphoglucose isomerase
Glucose -6-p ⇔ Fructose – 6- p
phosphoglucose isomerase
Factors affecting the enzyme activities
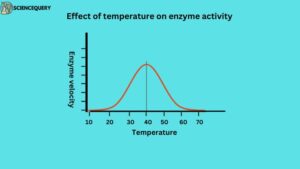
1. Effect of temperature
Enzyme reactions speed up as the temperature rises until reaching a maximum, then slow down. Optimum activity occurs at a temperature around 37.5ºC, which is the typical temperature for enzymes in human cells. Above this temperature, the enzyme structure begins to break down because enzymes are proteins and hence are denatured by heat. The optimum temperature of the enzyme is between 35 ºC – 45 ºC. Few enzymes are active even at 100 ºC. for example DNA polymerase, mutase adenylate kinase, etc.
So increase in temperature results in higher activation energy of molecule collision and interaction for the reaction to proceed faster.
A bell-shaped curve is usually observed. The majority of enzymes become inactive at a higher temperature above 70 and at lower temperatures. That is why food can be preserved in refrigerators at low temperatures to reduce bacterial enzyme activity.
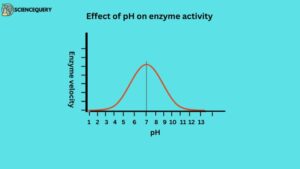
2. Effect of pH
pH means a quantitative measure of acidity.
An increase in the hydrogen ion concentration considerably influences enzyme activity.
A bell-shaped curve is normally observed. Each enzyme has optimum pH at which the velocity is maximum. Below and above this pH, the enzyme activity is much lower and at extreme pH, the enzyme becomes totally inactive. Changing the pH will affect the charge on the amino acid molecules. Amino acids that attracted each other may no longer be again the shape of an enzyme along with the active site will change. extremes of pH will also denature the enzymes.
The optimum pH for an enzyme depends on where it normally works. For example, enzymes in the small intestine have an optimum pH of about 7.5 but the stomach enzymes have an optimum pH of about 2. Most of the enzymes function within a working pH range of about 5-9 with neutral pH of 7 being the optimum.
3. Concentration of enzyme
As the concentration of the enzyme is increase the velocity of the reaction proportionally increases.
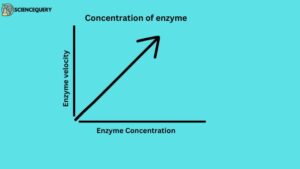
Enzyme concentration means the amount of enzymes present in a reaction is measured by the activity it catalyzes
4. Concentration of substrates
Substrates concentration is the amount of substrate present that can be turned into products. The concentration of substrates affects enzyme activity the rate of an enzyme-controlled reaction is affected by the concentration of the substrate. the rate of enzyme reaction increases with increasing substrate concentration. but at higher concentrations enzyme molecules become saturated so adding more substrate does not make much difference.
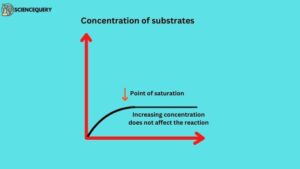
5. Enzyme inhibitors
Enzyme activators are molecules that bind to enzymes and increase their activity. And Enzyme inactivators are molecules that bind to enzymes and decrease their activities.
Sometimes inhibitors are attached to enzymes by a covalent bond. In this type of attachment, the inhibitors don’t go back to their original form that is we can say that it becomes irreversible because the inhibitor-enzyme bond is so strong that the inhibition can not be reversed by the addition of excess substrate.
Many enzyme inhibitors however attach to enzymes by weak interaction (use hydrogen bond) this also causes the inhibition but is reversible due to weak bonding.
There are two kinds of inhibitors
- Competitive inhibitors
It has a similar structure to normal substrate molecules and can fit into active sites of enzymes that is it competes with substrates for active sites. this can be overcome by increasing the concentration of substrates.
- Non-competitive inhibitors
They do not directly compete with substrates. Instead, it binds to other parts of the enzyme molecule and changes the shape of the whole enzyme. so, the inhibitors act to physically block the normal active sites.
Feedback inhibition
It controls enzymatic activity in a fair way. In the feedback mechanism, there is a second bonding site on the enzyme where the inhibitor binds. The absence or presence of inhibitors on second binding sites activates or deactivates the enzymes. this inhibitor is usually the product of a reaction formed during the metabolic pathway.
Models of enzymes
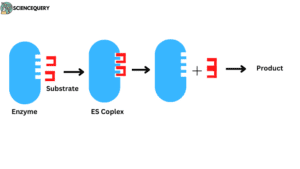
1. Lock-key model
Given by Emil Fisher in 1890. Enzymes are proteins and according to this model, a specific key can open a specific lock in the same manner a specific enzyme can transform one substrate into a product. According to this model, the active site has a rigid structure. there are no modifications or flexibility in the active site before, during, or after enzyme action.
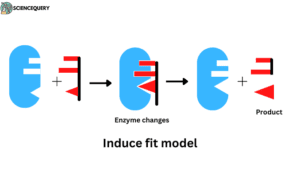
2. Induce fit model
Koshland 1959 proposed this model. The enzyme molecules are in an inactive form to become active they must undergo a slight change in the structure to a more specific form in order to accommodate a substrate.
the active site can be slightly modified as the substrate with the enzyme. this can be imagined in terms of the hand-and-glove relationship.
References
Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
