Introduction
The following topic is about enantiomers vs diastereomers and their definition and structure with a simple basic difference. Enantiomers and diastereomers are both optically active except for geometrical isomers. Enantiomers are separated by chromatography and diastereomers are separated by fractional distillation or crystallization.
What are enantiomers
Definition
Optical isomers which are non-superimposable mirror images of each other are called enantiomers and the phenomenon is known as enantiomerism.
- Non Superimposable means, when isomers are placed on top of each other they will not cover each other.
- Optical isomers means they have the same molecular and structural formula but they show different optical activity.
- Optical activity is an important feature of compounds with asymmetric carbon atoms.
- Enantiomers word made with Enantio + mer. Where “Enantio” means opposite and “mer” means unit. These are pairs of optical isomers, having opposite values of optical rotation.
- Enantiomers rotate in polarized light. If they rotate at the right angle it’s called dextrorotatory (d+).
- If they rotate in a left angle it’s called laevorotatory (L-). These isomers are called optical isomers.
- The compounds have identical molecular formulas but different structures are called isomers. The phenomenon of isomers is called isomerism.
Sugar shows five types of isomerism which are as follows
- Ketose-aldose isomerism
- D and L isomerism
- Optical isomerism
- Epimerism
- Anomerism.
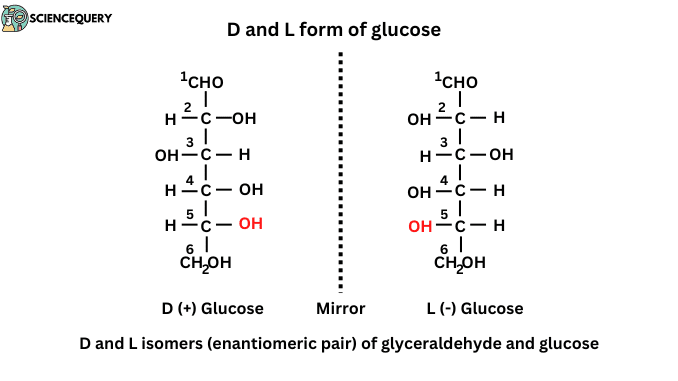
Enantiomers are referred to as D and L isomerism. D and L isomerism depends on the orientation of the H and OH groups around the asymmetric carbon atom.
E.g. Carbon atom number 5 in glucose determines whether the sugar belongs to D or L isomer.
Identification of Enantiomers
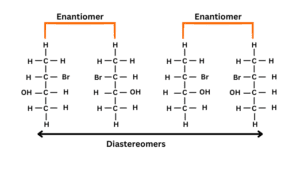
In two isomers two chiral centres are different, they will be identified as enantiomers.
D-series and L-series:
- The OH-group on asymmetric carbon atoms is on the right; it belongs to the D-series. ‘D’ means dextrorotatory.
- When it is on the left, it is a member of the L-series. ‘L’ means Laevorotatory.
- L and D forms are chemically and physically distinguishable. They have a different activities, melting points, and spectra.
- The structures of D and L-Glucose are based on the reference monosaccharide, D and L glyceraldehyde, a three-carbon sugar.
- D and L isomers are mirror images of each other. These two forms are called enantiomers. Most of the monosaccharides in living beings belong to the D-series.
- Enantiomers have different biological properties. For example, D(+) glucose gives CO2 and H2O energy through cellular respiration. L(-) Glucose can not be metabolized.
D(+)-Glucose → CO2 + H2O & energy
L(-)-Glucose → Not metabolized
Examples of Enantiomers
D and L forms of Glucose
Structure

D and L forms of Glucose are enantiomeric pairs. These forms are referred to as the position of H and OH groups around the asymmetric carbon.
D and L forms of glyceraldehyde
Structure
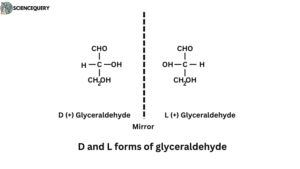
- Glyceraldehyde can exist in two configurations that are not superimposable and mirror images of each other.
- The second carbon atom of glyceraldehyde is linked to four different groups ( H, OH, CHO, and CH2OH ).
- This second carbon atom is known as asymmetric or chiral carbon. This carbon atom is responsible for two isomers of glyceraldehyde.
- If the four groups bonded to a carbon atom are all different, as in glyceraldehyde, then two possible configurations exist that cannot be superimposed on one another.
- These two isomers rotate plane‐polarized light in opposite directions and, thus, are said to be optically active.
What is diastereomers?
Definition
Diastereomers are stereoisomers that are not mirror images of each other. All geometrical isomers are diastereomers. They can be separated by fractional distillation or fractional crystallization.
- The term diastereomers is used to represent the stereoisomers that are not mirror images of one another.
- They are stereoisomers and have the same chemical formula. Monosaccharides (ex. Glucose, Mannose etc.) show stereoisomerism.
- Stereoisomerism is an important characteristic of monosaccharides.
- Organic compounds that have the same structural formula but differ in their spatial configuration are called stereoisomers.
- Diastereomers need not contain more than one chiral atom.
- Epimers are diastereomers that contain more than one chiral carbon and differ in the configuration of only one asymmetric carbon.
Identification of diastereomers
In two isomers one carbon centre is the same and one is different and is identified as a diastereomer.
Examples
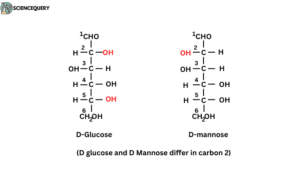
- Fumaric acid and malic acid, D-glucose, D- Mannose, D-galactose and other members of aldohexose are diastereoisomers.
- They have the same chemical formula and the same sequence of connectivity of atoms but differences in the spatial arrangements of some groups.
- We can identify the possible isomers of any compound through the number of asymmetric carbon atoms. Glucose contains 4 asymmetric carbon and thus has 16 isomers.
Structure of diastereomers
D-glucose and D-Mannose differ in carbon 2
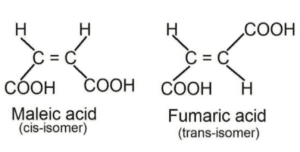
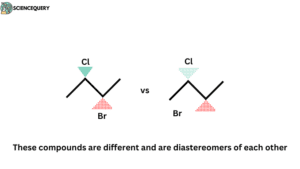
cis and trans isomers are diastereomers: For example the structure of maleic acid and fumaric acid.
Differentiate between enantiomers and diastereomers
Key points |
Enantiomers |
Diastereomers |
Definition |
These types of stereoisomers are mirror image and non super imposable compounds. | These types of compounds are non mirror image and non super imposable to each other. |
Physical properties |
They have the same physical properties except optical conditions. | Have different physical properties. Such as melting points, solubility in a given solvent, density, detective index etc. |
Chemical properties |
Identical chemical properties in achiral conditions. | Different chemical properties. |
Chiral centre |
Enantiomers have one or more chiral centres | Diastereomers have chiral or achiral centres. |
Optical activity |
Optically active. They rotate the monochromatic light in a right angle or left angle.
If the right angle rotates is called dextrorotatory and the left angle rotates its called laevorotatory. |
Are also optically active except for geometrical isomers. |
Separation method:- |
Enantiomers can not be separated by usual methods like fractional distillation because they have identical properties.
They are separated by chromatography. |
Diastereomers have different properties so they are separated by fractional crystallization. |
Rate of reaction |
The rate of reactions of enantiomers with optically inactive reagents (like HBr, H2SO4 etc.) are the same but with optically active reagents, the rate of reactions is different. | Rates of reactions of diastereomers with chiral or achiral are generally different. |
Q&A
1. What are enantiomers and diastereomers?
Enantiomers are the isomers which are non Superimposable mirror images and diastereomers, which are not mirror images and not Superimposable each other.
2. Diastereomers vs enantiomers vs meso?
Enantiomers are optically active and diastereomers are either optically active or inactive but meso compounds are actively inactive due to internal compensation.
3. How to differentiate between enantiomers and diastereomers?
Enantiomers are those that have identical physical or chemical properties but diastereomers have different physical or chemical properties.
4. How are enantiomers different?
Enantiomers are different from other isomers because they are optically active compounds and have opposite values of optical rotation
5. How are enantiomers separated?
Due to identical properties, they can not be separated by any usual method. They are separated by chromatography.
Conclusion
- Non-superimposable mirror images of isomers are called enantiomers.
- Enantiomers are optically active. They rotate in a plain polarized line in the right or left direction.
- If they rotate in the right direction they are referred to as the D-series. D-series is dextrorotatory.
- If they rotate in the left direction they are referred to as the L-series. L-series is laevorotatory.
- D and L isomerism depends on the position of the H and OH groups around the asymmetric carbon or chiral carbon.
- Carbon which is surrounded by four different groups is called chiral carbon or chiral centre.
- Enantiomers have identical physical properties such as melting point, solubility, densities etc.
- Enantiomers have different biological properties. D(+)-Glucose gives CO2, H2O and energy through glucose metabolism.
- Enantiomers have the same chemical properties except reactivity towards optically active reagents.
- D-glucose and L-Glucose, D-Lactate and L-Lactate, D-Glyceraldehyde and L-glyceraldehyde are examples of enantiomers.
- Stereoisomers, which are not enantiomers. That means they are not mirror images and not superimposable to each other.
- Stereoisomers have the same structural formula but differ in the spatial arrangements of connecting groups.
- Fumaric acid and Malic acid, glucose and mannose are examples of diastereomers. They have the same chemical formula but differ in one chiral centre.
- Diastereomers have the same chemical structure but differ in only one chiral centre. They are either optically active or inactive.
- Enantiomers and diastereomers are both optically active except for geometrical isomers.
- Enantiomers are separated by chromatography and diastereomers are separated by fractional distillation or crystallization.
References
Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
Essentials of Biochemistry, Pankaja Naik
Biochemistry 4th edition, U. Satyanarayana & U. Chakrapani
