Chromatography Definition
Chromatography is a technique invented by a Russian botanist Michael Tswett in 1906. It is the technique for the separation of colored substances into individual compounds. The name CHROMATOGRAPHY in Greek means CHROMA Colour and GRAPHY Writing. Chromatography definition is, therefore, a technique for the two phases are either the two defined below
- A solid stationary phase with a liquid or gaseous mobile phase
- A liquid stationary phase is a liquid or gaseous mobile phase
Theory of Choramotagraphy
It is based on the distribution of solutes between solvents that is a mobile and a stationary phase.
Distribution ratio = Amount of solute in the solvent (Stationary)/ Amount of solute in the other solvent (Mobile)
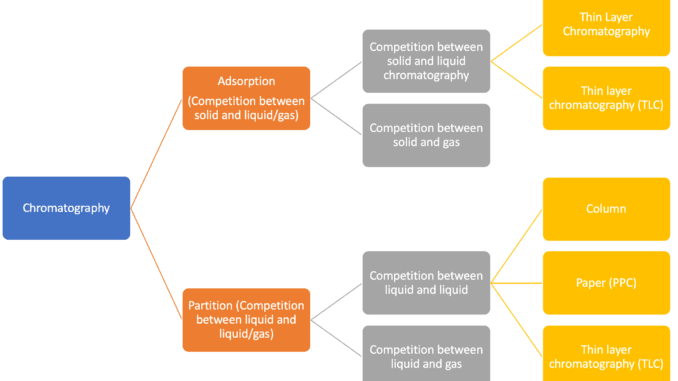
Classification of Chromatography
Adsorption chromatography
This chromatography technique is based on the difference in adsorption coefficients. In this method, a fixed phase is solid. the solutes are adsorbed in different parts of the adsorbent column. the adsorbed components are then eluted by passing suitable solvents.
Partition chromatography
In partition chromatography, the fixed phase may be a liquid strongly adsorbed on a solid which acts as a support. the solute gets distributed between the fixed liquid and the moving liquid (solvent). Paper chromatography is, therefore, an example of partition chromatography.
Gas chromatography
As the name suggests when the moving phase is a mixture of gases, it is called gas chromatography or vapor phase chromatography.
