Introduction
In science, almost all quantities being measured have a specific unit or way to complete the measurement process. Some quantities are measured using a single parameter while some require multiple parameters to do so.
Concentration is one such term that plays a crucial role in the field of chemistry. Through concentration, one can produce chemicals containing various properties and reactivity. If we maintain the concentration of a solution or its elements, we can obtain desired properties of the solution. When the concentration changes the properties of the solution may change as well.
Concentration is the ratio of the quantity of solute to that of the corresponding solution or solvent. One can define the units of concentration in various manners. It depends upon the properties and context of the substances involved.
Molarity and Normality are such units and describe the concentration of the solution. It depends upon various factors and we shall know more about these units in the article.
Some common ways to define the concentration of a solution are :
- Mass Concentration
- Volume Concentration
- Molar Concentration
- Molal Concentration
- Percentage Concentration
- Normal Concentration
These units of concentration are defined based on the factors like mass, volume, number of moles, percentage of solute, etc. Percentage concentration also has two more types, mass percentage and volume percentage in this mass and volume percentage of solute is calculated.
Definition
This section will tell us about the basic meaning and definition of the terms Molarity and Normality.
Molarity
Molarity is defined as the number of moles of solute dissolved in one liter (L) of solution.
It is generally denoted by ’M’
The unit of molarity is moles per liter (mol/L) or moles per cubic decimeter (mol/dm³).
Molarity is commonly used to describe the concentration of a solute in a solution and is an essential concept in various chemical calculations, including stoichiometry, dilutions, and determining reaction rates.
For example, if you have 0.5 moles of solute dissolved in 2 liters of solution, the molarity would be 0.25 M. Similarly, if you have 10 moles of solute in 500 milliliters (0.5 liters) of solution, the molarity would be 20 M.
Normality
Normality which is denoted as ‘N’, is a unit of concentration used in chemistry and particularly in acid-base reactions. It is the measure of reactive entities (such as ions or groups) constituting the ability to undergo various chemical reactions, per liter of the solution.
For example, in the case of a monoprotic acid (an acid that donates one proton per molecule), if you have 0.1 moles of acid dissolved in 1 liter of solution, the normality would be 0.1 N. However, if the acid is diprotic (donates two protons per molecule), the normality would be twice that value, i.e., 0.2 N.
Difference Between Molarity and Normality
After knowing about the basics of these two measurements of concentration, some questions may arise in your mind like, instead of two measurements why not use only one method, why there is a need for two measurements, what are the differences between them?
As both are measurements of concentration, they have various advantages and disadvantages which makes them different from each other. Hence, in order to have the knowledge of how and when these measurements are utilized we should know about the differences between them.
Measurement of concentration which is defined by the number of moles per liter of the solution.Measurement of concentration which is defined by the number of reactive entities per liter of solution.
Used for solutions that mainly involve the transfer of molecules specifically used for acid-base reactions and also for reactions that involve the transfer of protons(H+).
| Molarity | Normality |
|
|
|
|
|
|
Applications
Molarity
- Stoichiometry: The quantities of reactants and products involved in a chemical reaction are calculated using this method, hence it is extensively used in stoichiometric equations. It allows for the calculation of the number of substances needed or produced based on their molar ratios.
- Solution Preparation: With the help of Molarity one can accurately prepare the desired solution, as it is very important to maintain the appropriate concentration of every reactant of the solution. As molarity is considered the number of moles per volume of the solution, we can also get the exact volume of a reactant in order to prepare the solution.
- Titration: Titration is the process of finding the concentration of a solution with the help of another solution with known molarity. Hence, molarity is very important for this process.
- Reaction Rate: Molarity is often involved in the study of reaction rates. The rate of a reaction can be expressed in terms of changes in molarity per unit of time, providing insights into the kinetics of the reaction.
Normality
- Acid-Base Reactions: The stoichiometry and the quantity of the acid-base species are calculated with the help of normality. It is used and is also preferred for acid-base reactions, as it mainly deals with the number of reactive entities only, that is in this case the proton accepting or donating groups.
- Redox Reactions: Some specific redox reactions require Normality as the concentration measuring method, as the reaction involves the transfer of electrons and equivalents. Redox reactions are reactions in which reduction and oxidation both take place simultaneously.
- Equivalent Weight Calculations: Normality is used for calculating equivalent weights in various chemical reactions. Equivalent weight is the weight of a substance that is equivalent to one mole of reactive entity involved in the reaction.
- Analytical Chemistry: Analytical chemical techniques such as volumetric analysis uses the method of Normality, which is utilized for precise determination of the concentration of certain species in a sample.
How to Calculate?
So far we discussed the theoretical part of both Molarity and Normality, in this section, we will know about the mathematical way to determine the values and the expression used to calculate and obtain Molarity and Normality for a particular solution.
Molarity
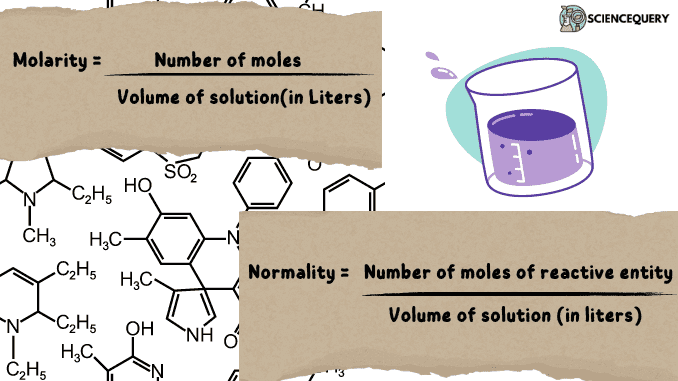
As we know Molarity is the number of moles per volume of a given solution and hence the respective mathematical expression comes out to be:
M = number of moles / volume of solution(in Liters)
Another popular expression used for the calculation is :
M = number of moles * 1000/ volume of solution(in mL)
One can find the number of moles by calculating the ratio:
Number of moles = Given mass/ Molar mass
Hence, by combining these equations we get a final equation:
M = (Given mass/ Molar mass)*(1000/ volume of solution(in mL))
Normality
Normality deals with the quantity of reactive groups and hence the formula then forms to be:
N = (number of moles of reactive entity) / Volume of solution (in liters)
Or
N = (Gram equivalent of solute) / Volume of solution (in liters)
Or N = (weight/equivalent weight) / Volume of solution (in liters)
Where, Equivalent weight = Molar mass / n
n= equivalents formed
Q&A
1. Why molarity and normality of HCl is equal?
HCl is a monoprotic acid. That is one molecule of HCl can donate a single proton. And hence the number of protons released by HCl in an aqueous solution becomes equal to the number of moles of it. This results, in the values of molarity and normality being equal.
2. why use normality instead of molarity?
Normality is used in place of molarity as some reactions involve the transfer of protons or equivalents. In order to deal with such reactions molarity cannot be preferred.
3. What is molarity and normality?
Molarity is a method of finding the concentration of a solution. This is calculated as the number of moles per volume of solution. Whereas, Normality is also a method to determine concentration but uses the number of reactive groups or equivalent weight for the calculation.
4. When are molarity and normality the same?
In the case of monoprotic acid or monoprotic base compounds, the normality and molarity of the solution are equal.
5. When to use normality vs molarity
Normality is used when a reaction involves the formation of reactive entities, whereas Molarity is used when there is a transfer of moles taking place in a solution.
6. Is molarity the same as normality?
Molarity and Normality both are methods to calculate the concentration of a solution but they use different parameters for the purpose, which makes them different from each other.
Reference:
https://www.sciencedirect.com/book/9780443191749/basic-life-science-methods
