Introduction
Functional groups of carbon are groups of elements that on adding to a carbon chain or elemental chains like that of a nitrogen or similar atoms, changes the property of the particular chain. By adding these functional groups one can achieve the synthesis of a completely new compound, which has different structures, physical and chemical properties. This article is about two functional groups of carbon and their comparison that is Aldehydes vs Ketones.
Aldehydes
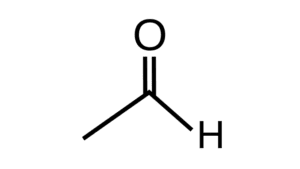
- Aldehydes are formed by the bonding of a carbonyl group with an aliphatic or aromatic carbon chain.
- The Carbonyl group consists of carbon having a double bond with an oxygen atom. Since the valency of carbon is 4 therefore after the oxygen double bond there remain 2 more free electrons on the carbon atom to be bonded. Out of which one is bonded with the valence electron of hydrogen while the other is bonded with the valence electron of another carbon atom from the carbon chain.
- Hence, due to the structure of the aldehyde group, the general chemical formula forms out to be ‘R-CHO’. Here ‘R’ represents the alkyl group and -CHO is the aldehyde functional group.
- In the nomenclature of carbon compounds when an aldehyde group is added, the suffix – ‘al’ is used. Firstly the name of the parent carbon chain is mentioned then the suffix ‘al’ is added.
- The Alkyl group is the chain of parent carbon which has one valence electron that gets bonded to other elements or functional groups.
- Aldehydes have a keen distinctive odor and are highly reactive in nature, that is their reactions require less amount of energy and are vulnerable to any sort of bond breakage and formation. Formaldehyde (CH2O), acetaldehyde (CH3CHO), and benzaldehyde (C6H5CHO) are some of the commonly used aldehydes.
- The applications of aldehydes are very vast and are used in multiple industries and for the manufacturing of various kinds of chemicals, polymers, materials, and some specific kinds of processes regarding exothermic or endothermic reactions.
- Depending upon the properties of aldehydes they can be found in different states that is, at room temperature, they can be solid, liquid, or gas. This property of aldehydes can be determined by the molecular interaction of the particles.
- For example, Formaldehyde is found in a gaseous state at room temperature while aldehydes like Acetaldehyde and Benzaldehyde are liquid at room temperature.
Ketones
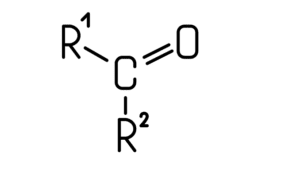
- Another kind of functional group is ketones. Ketones are formed by the bonding of a carbonyl group with two carbons of an elementary chain.
- In this, the valence electrons from the carbonyl carbon make a covalent bond with the valence electron of carbon of the elementary chain. Due to this the carbonyl group consists of two free lines which are meant to be bonded with other carbons.
- Hence, for this reason, the structure of ketones forms to be in such a way that its general formula is ‘R-CO-R’, where R and R’ are two different alkyl or aryl groups, while -CO- is the ketone group.
- Various biological processes involve ketones as the main reactant. Generally, ketones are easily found in nature, but they are also synthesized industrially. The range of applications includes solvents, in the production of plastics, resins, and pharmaceuticals, as well as in the fragrance and flavor industries.
- For the nomenclature of ketones suffix ‘-one’ is used in their IUPAC name with the parental carbon chain.
- Ketones formed by the alkyl group having less carbon, are soluble in water up to some extent. This occurs due to the presence of a polar carbonyl group.
- In organic solvents like ethanol, methanol, etc. they are easily solvable.
- The variation of the position of the carbonyl group in the carbon chain results in the formation of different isomers of ketones, and hence ketones show the isomerism property. For example, 2-pentanone and 3-pentanone are structural isomers.
Some Reactions of Aldehydes
1. Reduction reaction: Aldehydes are reduced using various reducing agents, strong reducing agents like LiAlH4, and NaBH4 are used in the reaction. Aldehydes are reduced to primary alcohols as a bi-product of the reaction.
HCHO + NaBH4 → CH3OH
As we can see that the reactant aldehyde on reacting with the reducing agent sodium borohydride gets reduced and the aldehyde gains hydrogen to form a primary aldehyde.
2. Oxidation reaction: On oxidizing aldehydes, we get carboxylic acids. Oxidizing agents like KMnO4, K2Cr2O7, and H2CrO4 are used for the reaction.
CH3CH2OH + K2Cr2O7 → CH3COOH
The reaction results in the loss of hydrogen and a gain of oxygen. In this the oxidation number of the active carbon increases.
3. Nucleophilic reaction: Due to the partial positive charge on the carbon of the carbonyl group, aldehydes become more reactive toward the nucleophiles. This causes lesser reaction time when reacted with nucleophiles.
HCHO + C2H5NH2 → H2C=NH + H2O
In this reaction, H2C=NH is an imine.
The aldehydes also get involved in a reaction that has great importance and significance as well, the reaction is known as Schiff Base formation reaction. In this reaction, aldehydes react with primary amines to form imines or when aldehydes are reacted with secondary amines they form enamines. It is commonly used in organic synthesis to create carbon-nitrogen bonds.
1. Aldol Condensation: Aldol condensation is a reaction in which aldehydes or even ketones form and enol is an intermediate product of the reaction which afterward reacts with other aldehydes or ketones.
HCHO + CH3COCH3 → CH3C(O)CH2OH
During the reaction, a carbon-carbon bond is formed. The product obtained is generally a β-hydroxy aldehyde. Acidic or basic conditions can be used to promote the aldol condensation reaction.
2. Cannizzaro Reaction: Aldehydes which do not contain α- hydrogen undergo Cannizzaro reaction. In this reaction, one molecule of the aldehyde is reduced to form a primary alcohol, while another molecule is oxidized to form a carboxylic acid.
HCHO + HCHO → HCOOH + CH3OH
The Cannizzaro reaction is typically facilitated by strong bases, such as hydroxide ions (OH-) or alkoxide ions (RO-).
3. Hemiacetal and Acetal Formation: The reaction of aldehydes with alcohol results in the formation of hemiacetals as well as Acetals.
Aldehydes react with an alcohol in the presence of an acid catalyst to form Hemiacetal. Further reaction with the alcohol and removal of water leads to the formation of an acetal. This reaction is commonly used for protecting aldehyde groups in organic synthesis.
Some Reactions of Ketones
1. Nucleophilic Addition
Nucleophiles such as water, alcohol, primary and secondary amines, etc when reacting with ketones result in the completion of nucleophilic reaction, with the carbonyl atom of the ketone. In this reaction, we get a tetrahedral intermediate. Further in the reaction protonation or elimination of a leaving group is executed.
For example, ketones react with primary amines to form imines or secondary amines to form enamines.
2. Reduction
Reducing agents like LiAlH4, NaBH4, etc. are used to reduce ketones. Ketones are generally reduced into secondary alcohols.
The carbonyl group is reduced to a hydroxyl group in the presence of these reducing agents. For example :
CH3COCH3 + NaBH4 → CH3CHOHCH3
Here, CH3CHOHCH3 is isopropanol.
3. Oxidation
Only strong oxidizing agents like KMnO4, H2CrO4, etc. can oxidize ketones, else in mild conditions they show an inert behavior towards the oxidation reaction. The carbonyl group is converted into a carboxyl group through this reaction.
CH3COCH3 + CH3OH + CrO3/H+ → CH3COOCH3 + H2O + CrO3
4. Aldol Condensation
Ketones can undergo aldol condensation reactions, similar to aldehydes. In the presence of an acidic or a basic catalyst, ketones react with other carbonyl compounds to form a β-hydroxy ketone or β-diketone. The reaction involves the formation of a carbon-carbon bond. The product obtained depends on the reaction conditions and the specific reactants used.
CH3COCH3 + C6H5CHO → CH3C(O)CH=C(C6H5)OH
5. Haloform Reaction
As the name suggests, the reaction involves the reaction of ketones in the presence of a strong base like NaOH along with oxidizing agents like I2 ( iodine), Cl2 (chlorine), etc. forming a compound known as Haloform.
Ketones with at least one methyl group adjacent to the carbonyl group can undergo the haloform reaction.
6. Wolff-Kishner Reduction
This reaction is used to convert Ketones into alkanes. The reaction involves the chemical process between ketones and hydrazine i.e. N2H4 in the presence of a strong base such as potassium hydroxide (KOH).
The carbonyl group is reduced to a methylene group, and the resulting compound is then dehydrated to form an alkane.
Uses and applications of Aldehydes
Aldehydes have various uses in the manufacturing industry, pharmaceutical industry, food industry, etc. Therefore, some of the popular uses are stated below.
- Many aldehydes have been explored which are popularly used in the production of flavors and fragrances like benzaldehyde is used in the production of almond flavorings, cinnamaldehyde provides the aroma and flavor of cinnamon.
- Formaldehyde is used as a preservative in the food industry. Glutaraldehyde is used as a disinfectant in food processing equipment.
- The anti-inflammatory drug naproxen is manufactured using an aldehyde intermediate
- Bakelite, which is a popular polymer in the industry is synthesized using formaldehyde.
- Aldehydes like formaldehyde and acetaldehyde are used as laboratory reagents and solvents.
Uses and applications of Ketones
The following are the uses of ketones:
- Methyl ethyl ketone and methyl isobutyl ketone are used as solvents in various industries, they are widely used in paints, coatings, adhesives, and cleaning agents.
- Polymer such as nylon, consists of cyclohexanone as an important ingredient in its production.
- Acetophenone is used in the making of flavors of cherry and almonds.
- Ketones are used for modifying the viscosity of various formulations like perfumes, lotions, and hair care products.
- Acetone is used as an analytical reagent and is used in various analytical techniques.
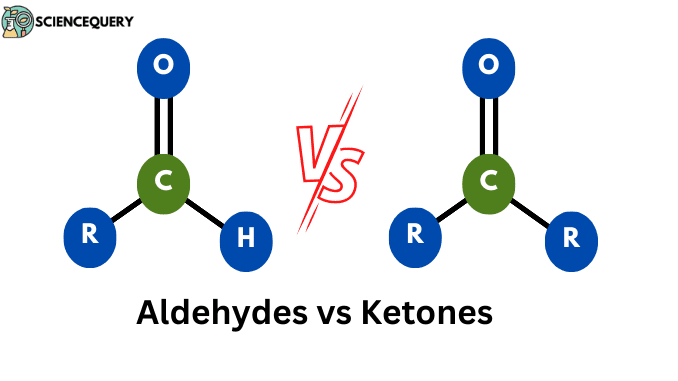
Aldehydes vs Ketones
Some of the basic differences between Aldehydes vs Ketones are as follows
Aldehyde |
Ketones |
| The carbonyl group is bonded with at least one hydrogen atom. | The carbonyl group is bonded with at least two carbon atoms. |
| Always placed at the end of the carbon chain. | Placed within the carbon chain. |
| The suffix used in IUPAC nomenclature is “-al”. | IUPAC nomenclature of ketone consists of the suffix “-one”. |
| More reactive due to the presence of a hydrogen atom. | Less reactive due to the carbonyl group is bounded to two carbon atoms. |
| Reduction and oxidation reactions are easily completed. | Ketones are inert to oxidation and reduction reactions at mild conditions. |
| Lower boiling points than ketones. | Have a high value of boiling points. |
| Examples – methanal, ethanal, etc. | Examples – acetone, cyclohexanone, etc. |
Q&A
1. Where are aldehydes found?
Aldehydes are found in various fruits, almonds, lemon, and oils, like cinnamon oil, etc.
2. How are aldehydes different from ketones?
The basic and most significant difference between aldehydes and ketones is hence in their structure.
The carbonyl group of aldehyde is bonded to at least one hydrogen and to a carbon of the elementary chain.
While the ketones consist of a carbonyl group bonded with two carbons of the parental chain.
3. Why aldehydes and ketones undergo nucleophilic addition?
Carbonyl group contains a partial positive charge on it. Because of this, they are capable of completing nucleophilic addition.
4. How are aldehydes and ketones named?
In the IUPAC nomenclature aldehydes are named by using the suffix “-al” in the name of the parental chain. While the suffix “-one” is used for naming a ketone compound.
5. What is aldehyde and ketone group?
Aldehyde is R-CHO where -CHO is the aldehyde group.
-CO- is the ketonic group of Ketone is R-CO-R’.
6. Why are ketones more stable than aldehydes?
Aldehydes consist of a hydrogen atom bonded with the carbonyl group, this causes the aldehydes to be more reactive. Since the aldehydes are easily reactive they become more unstable in nature.
7. Are aldehydes and ketones carboxylic acids?
No, the carboxylic group has the structure of the form “-COOH”, while aldehydes have the structure “-CHO” and that of ketones is “-CO-” . But with the help of oxidation reactions aldehydes and ketones can be converted into carboxylic acids.
Summary of Aldehydes vs Ketones
- Carbonyl groups bonded with an elementary carbon chain are used for aldehydes and ketones.
- Whenever in a carbonyl group a hydrogen atom forms a bond with the Carbon in it, we get a new functional group known as aldehydes.
- While in the case of ketones, the carbonyl group is bonded with two carbons of the parental chain.
- Aldehydes and ketones both undergo nucleophilic addition reactions due to the presence of a partial positive charge on the carbon of the carbonyl group.
- Reduction of aldehydes results in the formation of primary alcohols while that of ketones results in the formation of secondary alcohols.
- Aldehydes have a lower boiling point than ketones due to a possible hydrogen bond.
- Aldehydes and ketones are used in various industries which include the foods and flavors industry, the pharmaceutical industry, the cosmetic industry, the manufacturing of polymers, etc.
- Hence, aldehydes and ketones are of important functional groups and the compounds formed by them play a crucial role in completing the process of various industries.
Written By: Bharat Awasthi
References