Know in one minute about the Chemiosmotic hypothesis
|
Introduction
- The chemiosmotic hypothesis explains the mechanism of ATP Synthesis.
- It was given by Peter Mitchell in 1961.
- According to this hypothesis, the energy released from electrochemical potential is by the pumping of protons across the inner mitochondrial membrane.
- The energy in this electrochemical potential or proton motive force can be converted into ATP.
- Proton motive force is generated by the pumping of Protons.
- The energy released during electron transport is stored in the form of an electrochemical gradient of protons across the membrane.
- Proton means H+ ions and gradient means difference. So on the two different sites of a membrane, there will be some differences in protons.
- At one site the proton concentration will be much higher. In other sites, the H+ ion concentration will be much lower which will develop a gradient or difference.
- For maintaining equilibrium H+ ions will be moved from one site to another site and they will generate ATP from ADP & Pi.
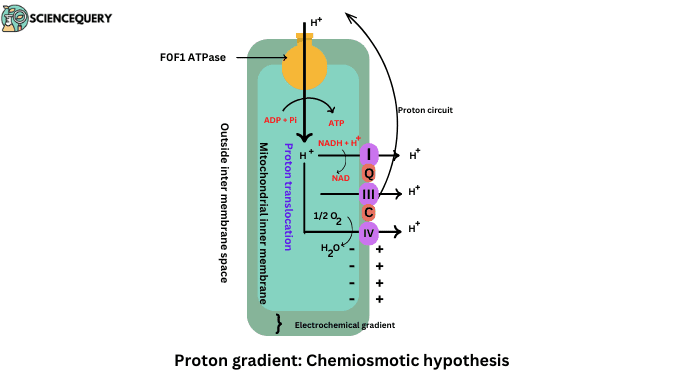
- Respiratory Complex I, Complex II, and Complex IV worked as a proton pump and generated electrochemical gradients.
- Protons are pumped out of the inner mitochondrial membrane to the matrix through the FoF1 complex. FoF1 complex is also known as ATP synthase enzyme.

ATP synthase is located in the inner mitochondrial membrane. It consists of two parts Fo and F1.
Fo particle:- it is embedded in the membrane. It involves the movement of the H+ proton from intermembrane space to matrix.
F1 Particles:- It is a head-like part that is projected from the surface of the membrane to a matrix.
It involves in synthesis of ATP by the combination of ADP + Pi → ATP
Chemiosmotic Synthesis of ATP
- According to the chemiosmotic hypothesis, the proton gradient or proton motive force is responsible for ATP Synthesis.
How to proton motive force generate
In excess amounts, Protons accumulate in the lumen of thylakoid and the inner membrane of mitochondria.
When H+ ion concentration Will be higher they move towards lower concentration through the ATP synthase enzyme.
Accumulation of Protons
In Chloroplast
During photosynthesis, the H+ ion accumulates in the lumen.
In Mitochondria
During cellular respiration, the proton accumulates in the inner membrane of the mitochondria and moves toward the matrix.
The inner mitochondrial membrane is impermeable to protons so their pumping results in the generation of the proton gradient.
ATP Synthesis in Chloroplast
ATP Synthesis reaction occurs in the thylakoid membrane of Chloroplast.
Protons accumulate in the lumen of thylakoid that’s why the proton gradient is established. Protons moved from the lumen to the membrane.
Factors that are involved in Proton transportation
- Plastoquinone
- FNR (ferredoxin NADP reductase),
- Photolysis of water or splitting of water molecules
Plastoquinone (PQ) : it is an electron transporter. Remove proton from stroma while electron transport.
PQ → PQH2
FNR: FNR brings protons from the stroma to the lumen of the thylakoid. This enzyme is responsible for the conversion of NAD to NADPH.
Photolysis of Water: During light reaction, H2O splits in oxygen, electron, and proton in Photosystem II. These hydrogen-positive ions transport to the lumen of the thylakoid
2 H2O → 4H+ 4e + O2
When proton Concentration is higher in the lumen of thylakoid they move to the membrane of the thylakoid membrane through ATP synthase enzyme. Where they bind with ADP and Pi and produce ATP.
The movement of Protons from higher concentration to lower concentration generates the Proton motive force which is responsible for the rotation of F1 particles.
What does the chemiosmotic hypothesis claim
The chemiosmotic hypothesis claims that the proton gradient is responsible for ATP Synthesis.
Experimental Proof of the chemiosmotic hypothesis
- It was given by A. Jangendory and B. Uribe in 1966. The process of ATP Synthesis takes place in the inner mitochondrial membrane and thylakoid membrane of Chloroplast.
- Thylakoid vesicles were isolated by these two scientists. Thylakoid membrane-bound with FoF1 particles. ATP synthase consists of these two particles Fo and F1.
- Thylakoid vesicles have these two particles. Firstly put these vesicles in a buffer solution (pH-4) until the same pH inside of the Vesicle. The pH of the buffer solution is four.
- When vesicles have 4 pH, put the vesicles in another Solution that has 8 pH, and ADP and inorganic Phosphate (Pi) is also present. Then ATP Synthesis will start.
- pH difference means variation in H+ ion concentration across the membrane that variation is responsible for the ATP Synthesis.
- So this experiment proved that the difference in proton gradient is responsible For ATP Synthesis.
- The process of synthesizing ATP from ADP and Pi coupled with ETC is Known as oxidative phosphorylation
Chemiosmotic model
- Chemiosmotic models explain the ATP Synthesis in the thylakoid of Chloroplast.
- These are the following steps of the chemiosmotic model which explained ATP Synthesis.
- Site of Proton accumulation:- Proton accumulates in the lumen of the thylakoid.
Proton accumulation
Through photolysis of water:- H2O split into proton electrons and oxygen. These protons are stored in the lumen of thylakoids. Proton concentration will be higher in the lumen.
Movement of the proton:- Proton moved from high concentration to lower concentration. In the lumen, pH is lower, and on the other side high pH approx. 7. This pH variation established the proton gradient or proton motive force.
ATP Synthesis:- Proton actively moved from ATP synthase. Movement of protons responsible for the rotation of the FoF1 complex. Their ADP and Pi convert into ATP.
Chemiosmosis Vs Oxidative Phosphorylation
Oxidative Phosphorylation |
Chemiosmotic hypothesis |
|
| Definition | Definition Synthesizing of ATP from ADP & Pi coupled with ETC is known as Oxidative Phosphorylation. | ATP molecules produced from proton gradients are known as chemiosmotic hypotheses. |
| Location in cell | ATP Synthesis occurs in the inner mitochondrial membrane. | ATP Synthesis occurs in the matrix of mitochondria. |
| Energy for ATP Synthesis | Energy is derived from the Oxidation of hydrogen acceptors like NADH2 and FADH2. They are formed in the Krebs cycle from the Oxidation of glucose | Energy derived from proton gradient for ATP Synthesis. |
| Involvement of electron transporter or electron transport chain | Electron transporters are involved in the binding of O2 to NADH2 or FADH2. | Electron transporters are not involved. Only plastoquinone involved |
| Proton motive force
Osmosis reaction |
Proton motive force not applied.
Osmosis reaction has not occurred. |
Proton motive force generated in ATP synthase enzyme.
Osmosis reaction is found. Protons moved from high concentrations to lower concentrations. |
Factors for the chemiosmotic generation of ATP
ATP synthase enzyme: ATP synthase enzyme activity is most important for ATP Synthesis.
Proton gradient: Proton gradient most important factor of ATP Synthesis.
Plastoquinone: Plastoquinone is an electron transporter. Plastoquinone transports protons into lumen.
Ferredoxin NADP reductase: It is responsible for the conversion of NAD to NADPH and for releasing protons into the lumen. It helps in proton accumulation.
Proton Concentration: Proton Concentration is the most important factor. High proton Concentration generates proton gradients and protons move from high concentration to lower concentration.
Q&A
What does the chemiosmotic hypothesis explain?
Chemiosmotic explains the mechanism of ATP Synthesis through the proton gradient.
What is the chemiosmotic theory of ATP Synthesis?
For the synthesis of ATP, the energy is released from the proton gradient by the pumping of protons across the inner mitochondrial membrane or thylakoid membrane. This process is called the chemiosmotic theory of ATP Synthesis.
What are the 3 steps of chemiosmosis?
These are the three steps of chemiosmosis:-
1. Accumulation of proton
2. Movement of proton and proton gradient generates
3. ATP synthesized
References
Karp’s Cell and Molecular Biology: Concepts and Experiments, 8th Edition
Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
Essentials of Biochemistry (for Medical Students). By Pankaja Naik, Shivananda Nayak B.
Biochemistry 4th edition, U. Satyanarayana & U. Chakrapani.
