Know in one minute about Chemisorption
|
Introduction
Chemisorption is a chemical phenomenon that is a type of adsorption. Adsorption is a process in which particles of one material get adhered to the surface of some other material, unlike absorption these particles do not penetrate through the surface of the material but just gets accumulated on its surface.
The particles which get accumulated are known as adsorbate, while the surface on which it gets accumulated is known as adsorbent.
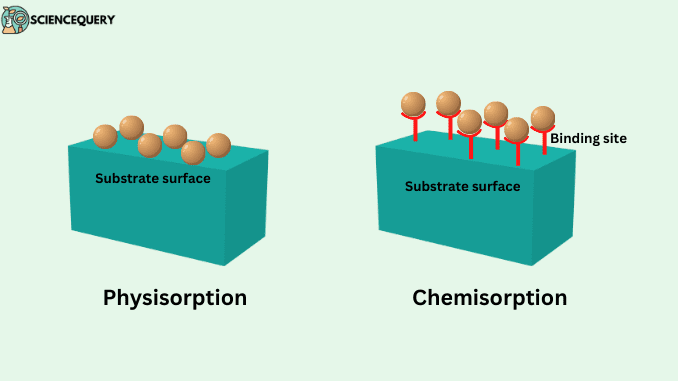
Based on the type of bond between adsorbate and adsorbent there are two types of adsorption phenomena:
- Physisorption
- Chemisorption
In this article, we will know more about Chemisorption.
Definition
Chemisorption is the process in which the adsorbate particle, as well as the adsorbent particles, are adhered to each other by the means of chemical bonds between them.
The process involves various sets of chemical reactions which result in the formation of new chemical bonds. The reactions are held between adsorbate and adsorbent due to which these particles are bonded to each other.
Chemisorption generally has high binding energy due to which sometimes it becomes irreversible in nature or it requires high energy to be reversed. This makes it an important phenomenon in various fields such as catalysis, surface science, and environmental science.
The chemical bonds may be covalent or ionic in nature. Due to the involvement of high-energy of activation, it is often referred to as activated adsorption (1).
Characteristics of Chemisorption
1. High specificity
Chemisorption is highly specific in nature. It occurs only when there is some possibility of chemical bonding between adsorbent and adsorbate. For example, oxygen is adsorbed on metals by virtue of oxide formation and hydrogen is adsorbed by transition metals due to hydride formation.
2. Irreversibility
As chemisorption involves compound formation, it is usually irreversible in nature. Chemisorption is an exothermic process but the process is very slow when the temperature is low as high activation energy is required for the process.
Generally, most of the chemical changes increase with the increase in temperature, likewise, chemisorption too increases with an increase in temperature. While a physisorption process may convert into chemisorption at high temperatures. Usually, high temperature is favorable for chemisorption.
3. Surface area
Since adsorption is a surface phenomenon, chemisorption depends upon the surface area of the adsorbent. As the surface area increases chemisorption increases as well.
4. Enthalpy of adsorption:
The enthalpy of chemisorption is high as it involves chemical bond formation. Its enthalpy is approximately 80 – 240 kJ mol-1 .
Diagrams and Equations
In order to know more about chemisorption and the equations involved during its process we must study some real-life applications of chemisorption.
1. Hydrogen Storage
One of the applications of chemisorption is the storage of hydrogen gas using metal hydrides, which involves the formation of chemical bonds between the hydrogen molecules and the metal atoms in the adsorbent material. The reaction can be represented by the following chemical equation:
Metal + nH2 → Metal hydride ( MHn)
Here, metal is the adsorbent material and H2 is the adsorbate which is being adsorbed on the metal to form metal hydride. The value n depends upon the stoichiometry of the metal hydride and the number of hydrogen molecules that can be adsorbed per metal atom.
The amount of hydrogen that can be stored is defined by a unit known as the Hydrogen storage capacity (HSC) of the adsorbent material. In order to calculate it a mathematical expression is used:
HSC = (MHn – M) / (2 * M) * 100%
Here, M denotes metal atoms.
2. Gas sensing
Chemisorption is also used in gas sensors, where the adsorbent is used to selectively adsorb certain gases. In gas sensing, chemisorption involves the adsorption of gas molecules onto the surface of a sensor material, resulting in a change in the electrical conductivity or optical properties of the material.
For example, metal oxides such as tungsten oxide can chemisorb oxygen gas, resulting in a change in the electrical conductivity of the material. This change can be used to detect the presence of oxygen gas in the surrounding environment.
The general equation involved in this process is :
A + MT → AMT
where A is any gas molecule, MT denotes sensor material and AMT denotes absorbed gas sensor material complex formed as a result.
3. Catalysis
In catalysis, chemisorption involves the adsorption of reactant molecules onto the surface of the catalyst, followed by the formation of chemical bonds between the reactant and the catalyst. This can result in the activation of the reactant and facilitate the chemical reaction to occur at a lower energy barrier. The chemical equation for a typical catalytic reaction involving chemisorption can be represented as:
Reactant + Catalyst → R-Cat
R-Cat denotes the formation of a compound in such a manner that the reactant molecules are adsorbed on the surface of the catalyst.
Some Sample Problems
In order to understand this topic well, we will now look at some problems related to chemisorption. Try to solve it by yourself. The solutions to these problems are mentioned below it.
1. Write any two characteristics of chemisorption.
Two characteristics of chemisorption are
- Surface area: As the surface area increases chemisorption increases as well
- Irreversibility: As chemisorption involves compound formation, it is usually irreversible in nature.
2. Why are powdered substances more effective adsorbents than their crystalline forms?
Powdered substances are more effective adsorbents than their crystalline forms because they have a much higher surface area available for adsorption. The surface area of a solid material is directly proportional to the number of adsorption sites available for the adsorbate molecules to interact with.
Q&A
1. What is the main difference between physisorption and chemisorption?
The key differences between them are:
- Whenever gaseous particles are accumulated on the surface of a solid and there is a weak Van Der Waal’s force between the molecules of adsorbate and the surface molecules of the adsorbent. Then the phenomenon being held is known as Physisorption.
- Physical adsorption at low temperatures may pass into chemisorption as the temperature is decreased. For example, dihydrogen is first adsorbed on nickel by van der Waal’s forces. Molecules of hydrogen then dissociate to form hydrogen atoms which are held on the surface by chemisorption.
- Chemisorption is the process in which the adsorbate particle, as well as the adsorbent particles, are adhered to each other by the means of a chemical bond between them.
- The process involves various sets of chemical reactions which result in the formation of new chemical bonds. The reactions are held between adsorbate and adsorbent due to which these particles are bonded to each other.
2. What is chemisorption?
It is a type of adsorption in which a chemical reaction occurs between the adsorbent and the adsorbate. Therefore, this chemical reaction involves the formation of chemical bonds between the adsorbent and the adsorbate. Thus, resulting in strong and stable adsorption.
The process of chemisorption is highly specific and selective, as it depends on the chemical properties of both the adsorbent and the adsorbate. This means that certain molecules will be more likely to undergo chemisorption than others, depending on their chemical structure and electronic properties.
3. How does chemisorption work?
Chemisorption takes place when the adsorbate and the adsorbent molecules interact with each other and result in a chemical reaction.
This chemical reaction or process requires a certain amount of heat to initiate. Hence it is preferred at high temperatures.
This chemical process may result in the formation of some different kinds of compounds. And due to this, the process becomes irreversible.
4. How is metal dispersion % calculated on pulse chemisorption micromeritics?
Metal dispersion is defined as the measurement of a fraction or part of the metal surface available for the catalytic reaction. Pulse chemisorption using micrometrics equipment is a common method for the measurement of metal dispersion.
The mathematical expression for calculating metal dispersion is:
Metal Dispersion (%) = (Number of metal atoms adsorbed / Total number of
Metal atoms) * 100
Written By: Bharat Awasthi
