Introduction
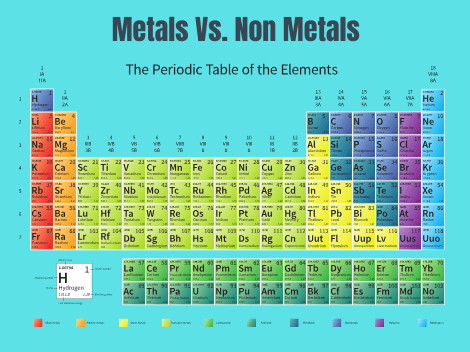
The periodic table contains a total of 118 elements so far. All of these elements are broadly classified as metal and non-metal on the periodic table. Besides these, there is another section of elements called as a metalloid. More than 75% at the left and middle part of the periodic table are metal. The elements on the right side of the periodic table are non-metals. From the sophisticated electronic devices, the study of metals has gained a lot of importance in daily life and their wide use. The metal and non-metal on the periodic table are discussed below (1) & (3).
Metals definition, examples, and properties
Definition of metals
Metals are those elements that are hard, and strong in nature. These are capable of bright, shiny, and light reflection. Also relatively heavy in weight and produces a metallic sound when hit. When reacts it usually produces alkaline oxides (3).
Examples of metals
Some examples of metals are silver, copper, gold, platinum, zinc, iron, aluminum, mercury, lead, magnesium, etc. All these metals are solid, except mercury which is liquid (3).
Physical properties of metals
1. Hardness
Most of the metals in the periodic table are hard, except sodium, potassium, lithium, etc. This metal is alkali metal. These metals are very soft and can be cut with a knife.
2. Malleability
Malleability is an important characteristic of metals. Most of the metals are malleable. That is, metals can be beaten into thin sheets with a hammer. Because of the property, iron is used in making big ships.
3. Strength
Most of the metals are strong and have high tensile strength. Some of the metals are not strong. Such as sodium, potassium, etc.
4. Ductility
It is another important property of metals. Metals are ductile. It can be drawn into wires. But all the metals are not equally ductile. Some metals are more ductile than others. Copper and aluminum metals are very ductile. Iron and magnesium are also quite ductile. Gold is the most ductile metal.
5. Density
Metals have high density and are very heavy. Iridium and osmium have the highest density and lithium have the lowest density.
6. Conduction
Metals have good conduction of heat and electricity. So the reason electric wires are made of metals like copper and aluminum.
7. Melting and boiling
Metals have high melting points and boiling points. Tungsten has the highest melting point and silver has a low boiling point.
8. Lustre
Metals have the quality of reflecting light from their surface. The property of metal having a shining surface is called “metallic luster”. The shiny appearance of metals makes them useful in making jewelry.
9. Physical state
Metals are solid at room temperature, except mercury. Most metals like iron, copper, aluminum, silver, etc. are solids at room temperature. Mercury is liquid at room temperature (1) & (3).
Chemical properties of metals
1. The reaction of metals with oxygen
The metal oxide is formed on the reaction of metals with oxygen.
Examples
a. Sodium forms sodium oxide when reacts with oxygen.
4Na + O₂ → 2Na₂O
Sodium oxygen sodium oxide
Lithium, potassium, sodium, etc. are known as alkali- metals. These metals react with oxygen.
b. Potassium forms potassium oxide when it reacts with oxygen.
4K + O₂ → 2K₂O
Potassium oxygen potassium oxide
Silver, gold, and platinum do not combine with the oxygen of air even at high temperatures.
2. The reaction of metals with water
Metals form hydroxide and hydrogen gas when it reacts with water.
Examples
a. Sodium forms sodium hydroxide and hydrogen gas along with a lot of heat when reacting with water.
Na + H₂O → NaOH + H₂
Sodium water sodium hydroxide hydrogen
b. Calcium forms calcium hydroxide and hydrogen gas when it reacts with water.
Ca + 2H₂O → Ca(OH)₂ + H₂
Calcium water calcium oxide hydrogen
c. Magnesium reacts with water and forms magnesium hydroxide and hydrogen gas.
Mg + H₂O → Mg(OH)₂ + H2
Magnesium water magnesium oxide hydrogen
Other metals do not react with water or very slowly react. Copper, silver, and gold do not react with steam.
3. The reaction of metals with dilute acid
When metals react with dilute acid then metal salt and hydrogen gas are formed.
Example:
a. Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas.
ZN + 2HCL → ZnCl₂ + H₂
Zinc hydrochloric acid zinc chloride hydrogen
b. Magnesium chloride and hydrogen are formed when magnesium reacts with dilute hydrochloric acid.
Mg + 2HCL → MgCl₂ + H₂
Magnesium hydrochloric acid magnesium chloride hydrogen
4. The reaction of metals with salt solutions
Displacement reaction is the reaction of metals with other metallic salt solutions. In this reaction, more reactive metal displaces the less reactive metal from its salt.
Example:
a. Iron reacts with copper sulfate solution and forms iron sulfate and copper.
Fe + CuSO₄ → FeSO₄ + Cu
Iron copper sulfate iron sulfate copper
b. Aluminum and zinc displace copper from the solution of copper sulfate.
2Al + 3CuSO₄ → Al₂(SO₄)₃ + 3Cu
Aluminum copper sulfate aluminum sulfate copper
Zn + CuSO₄ → ZnSO₄+ Cu + Cu
Zinc copper sulfate zinc sulfate copper
In all the above examples, iron, aluminum, and zinc are more reactive than copper. This is why they displace copper from its salt solution.
5. The reaction of metals with chlorine
Metals react with chlorine and form ionic chlorides. Metal chlorides have high melting points and boiling points. So, metal chlorides are non-volatile.
Example:
a. Sodium is a metal. It reacts with chlorine and forms an ionic chloride called sodium chloride.
2Na + Cl₂ → 2NaCl
Sodium chlorine sodium chloride
b. Magnesium on heating with chlorine readily forms magnesium chloride. It is an ionic chloride.
Mg + Cl₂ → MgCl₂
Magnesium chloride magnesium chloride
6. The reaction of metals with hydrogen
Most of the metals do not combine with hydrogen. Only a few reactive metals like sodium, calcium, potassium, and magnesium react with hydrogen to form metal hydrides. In a metal hydride, hydrogen is present in the form of a negative ion called a hydride ion.
Example:
When hydrogen gas reacts with sodium, then sodium hydride is formed.
2Na + H₂ → 2NaH
Sodium Hydrogen Sodium hydride
Hydrogen gas reacts with calcium and forms calcium hydride
Ca + H₂ → CaH₂
Calcium hydrogen calcium hydride (1) & (3).
Use of metal
In daily life, metals are used in various fields. Such as:
- Mercury is used in a thermometer to check the temperature.
- Aluminum and steel are used as utensils.
- Gold, silver, and platinum are used for jewelry and ornaments.
- Copper is used for making cable wires.
- Iron is used in automobiles.
- Iron and steel are used for construction purposes (3).
Non-metal definition, examples, and properties
Definition of non-metals
In chemistry, non-metals are the elements that have three properties of matter (solid, liquid, and gas) that are seen, which is neither bright nor shiny, cannot reflect light, are lightweight, and cannot conduct heat and electricity (3).
Examples of non-metals
Hydrogen, carbon, chlorine, nitrogen, bromine, neon, argon, sulfur, silicon, phosphorous, etc. are non-metals (3).
Physical properties of non-metals
1. Hardness
Most of the non-metals are generally soft. Sulfur and phosphorus are solid non-metals are quite soft. But one non-metal carbon (in the form of a diamond) is very hard.
2. Conduction
Non-metals cannot conduct heat and electricity. But some non-metals can conduct heat and electricity. For example, graphite and carbon non-metals can conduct heat and electricity.
3. Melting and boiling
Non-metals have low melting points and boiling points. But one non-metal has a high melting and boiling point. Diamond has high melting points and boiling points. The melting point of the diamond is, however, more than 3500°C, which is very high.
4. Lustre
Non-metals do not have luster. It means non-metals do not have a shining surface. The non-metals are dull. For example, sulfur and phosphorus have not lustered. That is they do not have a shining surface. But iodine is a non-metal, which has a shining surface.
5. Density
Non-metals have low densities are non-metals are light substances. Sulfur has a low density.
6. Ductility and malleability
Non-metals are neither malleable nor ductile. Non-metals are brittle, which means that non-metals break into pieces when hammered or stretched. For example, sulfur and phosphorus are solid non-metals that are non-malleable and non-ductile. But carbon is also a non-metal which is brittle.
7. Sonority
Non-metals are non-sonorous. They do not produce sound when they are hit by other objects.
8. Physical state
Non-metals are solids, liquids, and gases at room temperature. It can exist in all three physical states. These are solids, liquids, and gases. Sulfur, and phosphorus, are solid non-metals, bromine is liquid non-metal, and hydrogen, nitrogen, oxygen, etc. are gases non-metal.
9. Strong
Non-metals are not strong. They are easily broken. Graphite is not strong and it has low strength.
10. Colour
Non-metals have many different colors. For example sulfur is yellow, phosphorus is red, graphite is black, and hydrogen and oxygen are colorless (2) & (3).
Chemical properties of non-metals
1. The reaction of non-metals with oxygen
When non-metals react with oxygen it forms oxide.
Examples:
When carbon reacts with oxygen, carbon dioxide is formed along with the production of heat.
C + O₂ → CO₂ + Heat
Carbon oxygen-carbon dioxide
Sulfur gives sulfur dioxide when reacting with oxygen. Sulfur catches fire when exposed to air.
S + O₂ → SO₂
Sulfur oxygen sulfur dioxide
2. The reaction of non-metals with water
Non-metals do not react with water to evolve hydrogen gas. This is because non-metals cannot give electrons to reduce the hydrogen ions of water into hydrogen gas.
3. The reaction of non-metals with dilute acid
Non-metals do not react with dilute acids.
4. The reaction of non-metals with salt salutations
A more reactive non-metal displaces a less reactive non-metal from its salt salutation.
- When chlorine reacts with a solution of sodium bromide, then sodium chloride and bromine are formed
2NaBr + Cl₂ → 2NaCl + Br₂
Sodium bromide chlorine sodium chloride bromine
5. The reaction of non-metals with chlorine
Non-metals react with chlorine and form chloride.
- Hydrogen is a non-metal. When it reacts with chlorine it forms chloride, called hydrogen chloride.
H₂ + Cl₂ → 2HCl
Hydrogen chloride hydrogen chloride
6. The reaction of non-metals with hydrogen
Non-metal reacts with hydrogen and forms hydrides.
- Sulfur is a non-metal which combines with hydrogen to form a covalent hydride called hydrogen sulfide.
H₂ + S → H₂S
Hydrogen sulfur hydrogen sulfide (3).
Use of non-metal
- Oxygen is a non-metal.
- Chlorine is used for purifying water.
- Nitrogen is used by plants.
- Graphite is used in making leads
- Carbon is used as a fuel.
- Diamond is used in industries for cutting glass.
- Neon and argon are used in different types of lights (2).
Metalloids definition, examples, and properties
Definition of metalloids
In chemistry, an element that exhibits the properties of both metals and non-metals is called a metalloid (3).
Examples of metalloids
Boron, silicon, germanium, arsenic, antimony, tellurium, and polonium are metalloids. (3).
Physical Properties of metalloids
1. Physical state
Metalloids are solid at room temperature.
2. Lustre
Metalloids can be shiny or dull.
3. Conduction
Metalloids have low conduction of heat and electricity. That is metalloids are semiconductors.
4. Ductility and malleability
Metalloids are neither malleable nor ductile. Metalloids are brittle, which means that non-metals break into pieces when hammered or stretched.
5. Melting and boiling
It has a melting and boiling point.
6. Density
As compared to metals, metalloids have low density (3) & (5).
Use of metalloids
- Boron carbide is further used in pressure molding bulletproof jackets.
- Silicon has semi-conductive properties. So silicon is used in an application in computer chips.
- It is used in the form of boric acid and can be used as a cleaning agent.
- Germanium is used in semiconductor industries to improve the conductive properties.
- Antimony is used as one of the ingredients in paints and ceramic enamels. In ancient times, Egyptians used antimony as a cosmetic.
Difference between metal, non-metal, and metalloids (1) & (3).
Properties |
Metal |
Non-metal |
Metalloid |
||
| 1. Physical state | Metals are solid at room temperature | Non-metals are solids, liquids, and gases at room temperature. | Metalloids are solid at room temperature.
|
||
| 2. Density | Metals have high density and are very heavy. | Non-metals have low densities are non-metals are light substances. | As compared to metals, metalloids have low density.
|
||
| 3. Heat conduction | Metals have good conduction of heat. | Non-metals cannot conduct heat. | Metalloids have low conduction of heat. | ||
| 4. Luster | Metals have the quality of reflecting light from their surface. | Non-metals do not have luster. It means non-metals do not have a shining surface. The non-metals are dull. | Metalloids can be shiny or dull. | ||
| 5. Electricity conduction | Metals have good conduction of electricity. | Non-metals cannot conduct electricity. | Metalloids have low conduction of electricity. | ||
| 6. Melting and boiling point | Metals have high melting points and boiling points. | Most of the non-metals have low melting points and boiling points. | It has a melting and boiling point. | ||
| 7. Ductility | It is another important property of metals. Metals are ductile. It can be drawn into wires. | Non-metals are not ductile. | Metalloids are also not ductile. | ||
| 8. Malleability | Malleability is an important characteristic of metals. Most of the metals are malleable. That is, metals can be beaten into thin sheets with a hammer. | Non-metals are not malleable. Non-metals are brittle, which means that non-metals break into pieces when hammered or stretched. | Metalloids are not malleable. Metalloids are brittle, which means that non-metals break into pieces when hammered or stretched.
|
||
| 9. Oxide- water interaction | It forms a base when reacts with oxide-water | It forms acid | These compounds can form both base and acid. | ||
| 10. Nature of oxides
|
Metal formed basic oxide. | Non-metal formed acidic oxide. | Metalloids formed acidic oxide. | ||
| 11. Ionic tendency | Form cations | Form anions | Can form both | ||
Metal and non-metal on the periodic table
In 1829, German scientist Dobereiner first tried to decipher the origin of a few atoms with atomic mass and similarities between their religions. This is called the Law of Triads. In 1864 another scientist Newland also tried to decode the origin of a few atoms with the atomic mass and similarity between their religions. Russian scientist Mendeleev in 1869 has arranged elements incrementally according to the atomic mass, which is called Mendeleev’s periodic law. In the periodic table, metals are located on the left and middle parts. The elements on the right side of the periodic table are nonmetals. Besides these, there is another section of elements called metalloids (4).
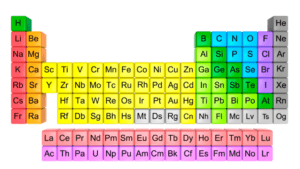
The periodic table of elements can be considered as a single document that reflects the behavior of elements in a simple and logical way. That means it is possible to predict the nature and behavior of the metals and non- metals only knowing the location of metals and non-metals in the periodic table.
In fact, the organization of metals and non-metal on the periodic table was a major advance in the history of chemistry. Elements that exhibit similar chemistry appear in vertical columns called groups (numbers 1-18 from left to right).
In the periodic table, there are seven horizontal rows which are called periods. In the periodic table, elements can be broadly divided into metals, non-metals, and metalloids. Metals are located on the left side of the periodic table and non-metals are located on the upper right. They are separated by a diagonal band of metalloids. In the periodic table alkali metals (group 1) and alkali earth metals (group 2) on the far left, and the halogens (group 17) and the noble gases (group 18) on the far right (6) & (4).
