Introduction
Strong Acids and Bases are those acids and bases which completely ionized into ions (H+ and OH- ions) in aqueous solutions.
Strong Acids
Before diving into the topic, let us discuss about Acids. Acids are substances that release H+ ions on dissociation in an aqueous solution or accept electron pairs. These are often sour in taste.
Definition
As we know, acid dissociates or gets ionized in their aqueous solution. Strong acids are those acids that completely get ionized into ions, (specifically H+ ion which is also commonly said as a proton) in their aqueous solutions.
pH range of strong acids is 0 to 3.
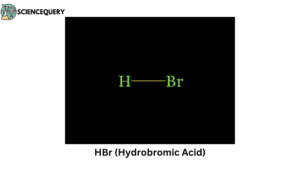
Examples
Some examples of Strong acids are





Problems related to strong acids
1. What is the pH range for strong acids?
The pH range for strong acids is 0 to 3.
2. What is the pH of HCl present in the human stomach?
As we all know HCl is a strong acid and it also helps to digest food in the human body. The HCl secreted in the human body has a pH ranging between 1.5 to 3.5.
3. How do Strong Acids dissociate in water?
Strong Acids like HCl, H2SO4, HNO3, etc. when comes in contact with water, due to their high dissociation tendency (or large value of the dissociation constant) they rapidly release H+ ions in the solvent. This results in a high concentration of H+ ions in the solution.
4. What are the characteristics of strong acids?
Some characteristics of strong acids are
- Strong acids react with active metals to produce hydrogen.
- Strong acids with strong bases produce salt and water.
- It turns the Universal color indicator to red.
- They are also responsible for some severe types of corrosion.
5. What happens when strong acids react with weak bases?
Strong acids when mixed with equimolar weak bases, they produce acidic salt.
Base(aq) + H₃O⁺(aq) → H(Base)⁺(aq) + H₂O(l)
6. How do Non-metals react with strong acids?
Non-metals usually do not react with acids. This happens because whenever a substance reacts with acids it provides electrons to acid and then H+ gets released.
Strong Bases
Introduction
Bases are substances that have a pH ranging from 7 to 14. Furthermore, they are also defined as compounds that donate electron pairs
Or
Substances that dissociate in water to give OH–.
Definition
Strong bases are substances that completely dissociate into OH– ions in their aqueous solution. These substances react with strong acids to give neutral salt. The pH of strong bases ranges between 12 to 14.
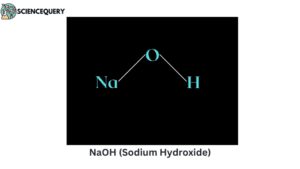
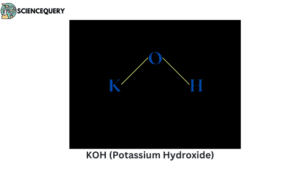
Examples
Let us now have a look at some examples of strong bases.

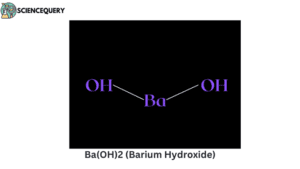
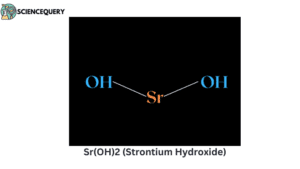
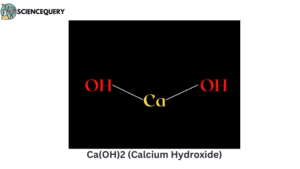
Problems related to strong bases
1. What is the pH range for strong bases?
Strong bases have a pH in the range of 12 to 14.
2. What is the pH of NaOH(sodium hydroxide or caustic soda)?
NaOH has a pH of 13 in its aqueous solution.
3. What happens to strong bases when they are mixed in water?
When strong bases like NaOH, KOH, LiOH, etc. are mixed in water it releases a large amount of OH– ions, which substantially results in the increase of concentration of OH– in the water solution.
4. State the characteristics of Strong Bases.
Characteristics of strong bases are
- They are very good conductors of electricity in their aqueous form.
- These substances have corrosive properties and can be very hazardous.
- They change the red litmus to blue.
- On reacting with strong acids they produce neutral salt.
5. What are the products when strong bases react with weak acids and strong acids?
When strong bases react with equimolar weak acids it gives basic salt as a product. On reacting with equimolar strong acids, it gives neutral salt
Q&A
1. What are strong acids and bases?
Strong acids are those substances that completely get ionized into ions, (specifically H+ ion which is also commonly said as a proton) in their aqueous solutions.
Strong bases are substances that completely dissociate into OH– ions in their aqueous solution.
2. How to identify strong and weak acids and bases?
The aqueous solution of both Strong acids and bases are very good conductors of electricity, as their individual aqueous solutions consist of ions that are released due to their dissociation.
3. Strong acids and bases belong to which class of chemical hazards?
Strong acids and bases are considered to be corrosive in the context of chemical hazards. This is because when any object comes in contact with them, then generally it gets corroded. Also when these chemicals come in contact with human skin, then the skin is severely damaged and in most cases the skin is non-recoverable.
Reference
NCERT Chemistry class 10th textbook.
Written By: Bharat Awasthi
