Introduction
Based on the complexity of structure there are three types of proteins. Conjugated protein is one of them it consists of simple proteins with some non-protein components. The non-protein group is known as the prosthetic group. Like other proteins, conjugated proteins have a specific structure and function which are discussed here (1).
Protein is the basic ingredient of food and is an abundant intracellular organic biomolecule with different polypeptide chains. Also called macromolecules. Conjugated protein is a type of protein and is classified according to the structure of the protein.
Conjugated protein is formed by combining simple proteins with a non-protein or chemical substance. These types of proteins are lipoproteins, glycoproteins, nucleoproteins, phosphoproteins, hemoproteins, flavoproteins, metalloproteins, phytochromes, cytochromes, opsins, and chromoproteins (3).
Conjugated protein
Conjugated proteins are a type of protein that contains some non-protein elements called the prosthetic group. This group can be separated from the protein part by carrying out hydrolysis very carefully. When a conjugated protein is attached to its prosthetic group it is known as apoprotein. These types of proteins are attached by covalent bonds or weak interactions (2) & (3).
Structure
Conjugated proteins are not only composed of amino acids. It is formed by binding some non-protein substance covalent bonds with amino acids.
The bond between the conjugated protein is not very strong. Such proteins are bound by covalent bonds. It contains an amino acid part combined with a non-protein substance like lipid, carbohydrate, etc. There are various types of conjugated proteins (2) & (4) these are as follows
Types of conjugated proteins
1. Nucleoproteins
- In these proteins, the prosthetic group is a nucleic acid.
- Nucleoproteins include ribosomes, nucleosomes, and viral nucleocapsid proteins.
- These types of proteins are mainly found in chromosomes.
- They are characterized by the formation of stable complexes.
- It has been prepared from the major organs of mammals, including the liver, kidney, brain, pancreas, etc.
- The nucleoprotein is found in the nucleus of the cell from which the specified cells originated.
Structure of nucleoproteins
Nucleoproteins are usually composed of residues of high-quality basic amino acids (lysine, arginine, and histidine). Each nucleoprotein has its own specific structure but they all contain these types of amino acids.
At physiological pH, these amino acids are positively charged, which supports the interaction with the molecules of the genetic material. Important techniques for determining the structure of nucleoproteins include X-ray diffraction, nuclear magnetic resonance. Nucleoproteins are classified according to the nucleic acids to which they are attached (4) & (3).
Example of nucleoproteins
Examples of nucleoproteins are nuclein, and nucleohistone from nuclei-rich material (glandular tissue). DNP is mainly present in all cell nuclei, especially in the chromosomes and also in the matrix of mitochondria and chloroplast.
It is associated with the storage and expression of genetic information of heredity. mRNA, tRNA is found in cytoplasm associated with the synthesis of proteins (1).
2. Chromoproteins
- It is a conjugated protein that contains a pigmented prosthetic group.
- In these proteins, a prosthetic group is a chromophoric group called the colored prosthetic group.
- Single chromoprotein acts as both phytochrome and a phototropin.
- They are characterized by an electron absorption band in the near-UV, visible, or near-IR spectral range (1) & (2).
Example
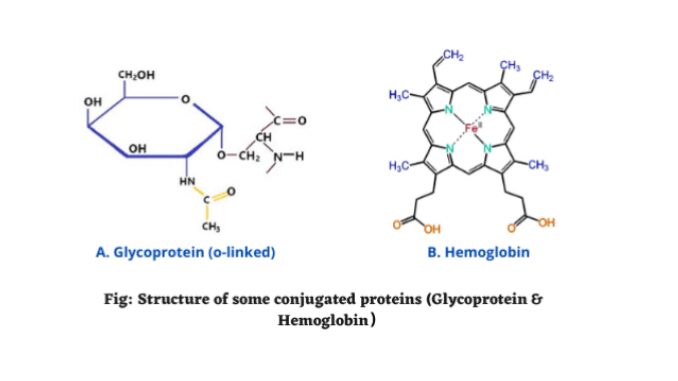
Examples of chromo proteins are the hemoglobin (blood of animals), hemocyanin (crustacean blood), cytochrome (mitochondria and plastids), and flavoproteins (mitochondrial membrane).
- Hemoglobin: The simple protein globulin is united with iron-containing pigment haem.
- Rhodopsin: The prosthetic group is a carotenoid pigment.
- Cytochrome: Conjugated with haem.
- Flavoproteins: Prosthetic group is a vitamin, riboflavin.
- Haemocyanin: The pigment part contains copper molecules (3).
3. Phosphoproteins
- This protein is attached by a single phosphate group or a complex molecule.
- They are soluble in alkali.
- These are proteins in combination with a phosphate-containing radical.
- Phosphoproteins are insoluble in water.
- In these proteins, the prosthetic group possesses phosphoric acid in some form other than in the nucleic acids or in the lipoproteins.
Structure
It is a conjugated protein. That means this type of protein is made of amino acids and some non-protein substances. A phosphate group is present in this protein. These proteins are attached to a single phosphate group or a complex molecule like 5-PHOSPAHTE–DNA.
Example
Examples of phosphoproteins are caseinogen (milk) and ovovitellin (egg yolk) (4) & (2).
4. Glycoproteins
- Glycoprotein is a type of conjugated protein that contains oligosaccharide chains.
- The carbohydrates or oligosaccharide chains are attached to amino acids by covalent bonds.
- They are located on the surface of the lipid bi-layer of cell membranes.
- These types of proteins are also called mucoproteins.
- They are the proteins having carbohydrate prosthetic groups and these on hydrolysis yield amino sugars such as hexoseamines.
- In most glycoproteins, there is a linkage between Asn, Thr, and Ser residue and N-acetyl hexosamines.
- Glycoproteins may be intracellular or secretory.
Structure
Glycoproteins are attached to the amino acid side chains. It contains glycan’s. Glycans are carbohydrate chains. It is saccharide polymer, which can attach to either lipids or amino acids. The bonds between glycoproteins are formed through a process known as glycosylation.
Example
These are found in ovomucoid in egg white and chondromucoid in cartilage. Egg albumins, some serum albumins, and certain serum globulins are also some examples of glycoproteins (1) & (3).
5. Lipoproteins
- They are soluble in water.
- Lipoproteins are conjugated with lipids, generally phospholipids.
- There are two types of lipoprotein in the cell membrane. These are low-density lipoprotein and high-density lipoprotein.
- They are the primary proteins of cell membranes.
- It is a group of proteins containing both lipids and proteins.
- Lipoproteins are larger and smaller dense when the fat-to-protein ratio is increased.
- They are macromolecular complexes.
- It is originated from the liver and intestine
Structure
These proteins are made up of fats surrounded by a single layer of phospholipids. They have a main hydrophobic core of non-polar lipids. This hydrophobic core is enclosed by a hydrophilic core. The hydrophilic core consists of phospholipids, cholesterol, and apolipoproteins. Apolipoprotein is a special type of protein that is attached to the outer shell of the hydrophobic layer.
Example
Examples of lipoproteins are many enzymes, structural proteins, antigens, toxins, blood plasma, blood corpuscles, etc. (2).
6. Metalloproteins
- Metalloproteins are conjugated proteins that contain metal. It is an integral part of the structure.
- Metals found in metalloproteins are basically iron, magnesium, copper, and manganese.
- It contains a metal ion cofactor.
- They are attached together by at least one metal ion.
- A large protein is part of this type of protein class.
Structure
Metalloproteins are attached to at least one metal ion. They use metal ions for a variety of biological purposes and are essential for life. Metal ions of metalloproteins are mixed with nitrogen, oxygen, or sulfur centers belonging to amino acid residues of the protein.
Example
Hemoglobin and chlorophyll are the major examples of metalloproteins. Hemoglobin is a metalloprotein found within RBCs (1).
Function or uses of conjugated proteins
1. Nucleoprotein plays an important role in biological functions such as transcription, translation and regulating gene expression, and regulating the metabolism of RNA.
2. Chromoprotein also regulates genes.
3. Hemoglobin is a chromoprotein that transfers oxygen in the blood from the lungs to the tissues.
4. Rhodopsin is a pigment-containing protein. It converts the light into an electrical signal. This type of protein allows the rod cells in the human eyes to absorb light and make it essential to vision in dim light.
5. Phosphoprotein is necessary to recruit the causes of protein growth in the ribosome.
6. Glycoproteins are involved in many physiological activities including immunity. They are the receptors of the cell.
7. Ovomucoid is a glycoprotein. It is an egg white protein with important anti-tryptic functions.
8. Lipoproteins also act as receptors. These proteins play an important role as drug receptors. The function of lipoproteins is to transport hydrophobic lipid molecules in water. These proteins carry cholesterol.
9. Metalloproteins play an important role in some biological processes such as photosynthesis, respiration, water oxidation, molecular oxygen reduction, etc. (1) & (3).
Q&A
1. What are conjugated proteins?
Based on the structure, there are three types of protein. Conjugated protein is one of them. When amino acids are attached to some non-protein substances then a protein is produced. It is known as conjugated protein.
2. Which conjugated proteins contain carbohydrates?
Glycoprotein contains carbohydrates. The carbohydrates or oligosaccharide chains are attached to amino acids by covalent bonds in these proteins.
3. What is a prosthetic group? identify the prosthetic group in the following conjugated proteins
A prosthetic group is a non-protein substance. It is part of the structure of the conjugated proteins. This non-protein substance is attached to an amino acid. These groups are attached to the proteins by a covalent bond.
| Conjugated protein | Prosthetic group |
| Glycoprotein | Carbohydrates |
| Lipoprotein | Lipids |
| Phosphoproteins | Phosphate group |
4. How are proteins conjugated into lipoproteins?
Proteins are conjugated into lipoproteins by adding a non-protein substance called lipids to amino acids.
References
1. B. Powar and G. R. Chatwal. Biochemistry, B. SC (general & honors course) and M. Sc. Himalaya publishing house, Chapter: Peptides and proteins. Page no: 192 to 206.
2. Chandrasekhar Chakrabarti. Modern approach to a textbook of core Zoology, General & Honours. Nirmala Library, A Publishing House under the Prestigious International Standard Book Number (ISBN) System. Kolkata, (India). Part – II. Chapter- Carbohydrates, protein, and lipid. Page: 2nd – 25- 21 to 2nd – 25 – 35.
3. Ajoy Paul. Zoology Honours, Volume- 1, Books & Allied (P) Ltd. Chapter: Proteins. Page no- 771 to 782.
