Introduction
The electron transport chain is one of the processes in cellular respiration. All the green plants synthesize carbohydrates via photosynthesis. This carbohydrate is further broken into simpler molecules.
Enzymes present in cell cytoplasm and mitochondria of animals are used during carbohydrate molecule breakdown. This process involves a variety of steps like glycolysis in cytoplasms and several steps occur in the mitochondria of the cell.
The Krebs cycle occurs on the outer lamellae of the mitochondria in the cell. Some steps are completed on the inner lamellae of the mitochondria. Here the oxidation of glucose produces water and carbon dioxide. The energy that is generated from the oxidation of glucose in the mitochondria of the cell is accomplished through a number of processes. One such process is the electron transport chain (1) & (3).
Definition of the electron transport chain
In the Krebs cycle, glucose molecules are completely oxidized. But energy is not released until NADH + H⁺ and FADH₂ are completely oxidized. NADH + H⁺ and FADH₂ do not combine directly with oxygen. The electron emitted from NADH + H⁺ or FADH₂ reaches oxygen through a series of electron carriers called the electron transport chain.
Although different compounds are oxidized in the two stages of the glycolysis and Krebs cycle, molecular oxygen is not required in any of the cases. Any compound is oxidized with a coenzyme such as NAD⁺ or FAD. As a result, the coenzymes are oxidized by themselves and converted into NADH + H⁺ or FADH₂. The coenzymes oxidize themselves with the help of some electron carriers and produce high-energy ATP molecules through the electron transport chain (3) & (6).
Features
Below are some of the features of the electron transport chain.
1. The electron transport chain is a series of electron carriers in the inner mitochondrial membrane of eukaryotic cells, through which the electrons obtained from the glycolysis and Krebs cycle are released.
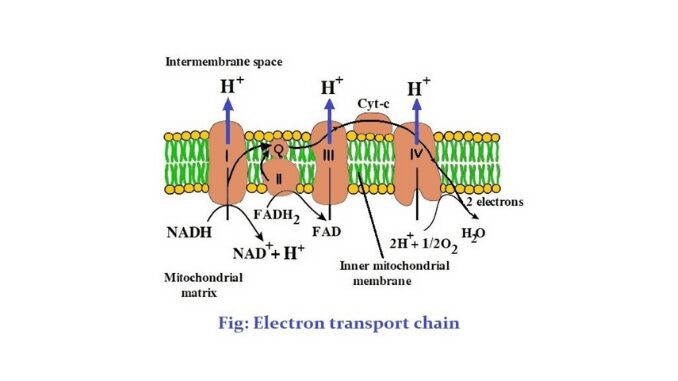
2. There are 5 complexes in this chain. These are complex I- NADH/NADPH: CoQ reductase, complex II- Succinate: CoQ reductase, complex III- Reduced CoQ (CoQH₂): cytochrome c reductase, complex IV- Cytochrome c oxidase, complex V- ATPase (ATP synthesizing system).
3. There are two mobile carriers, such as CoQ (Coenzymes Q) and cytochrome C.
4. The main carriers of the chain are cytochrome b (cyt b), cytochrome c (cyt c), cytochrome a (cyt a), and cytochrome a₃ (cyt a₃).
5. In the electron transport system, cytochromes are arranged according to their redox potential.
6. A pair of electrons are transported at each stage of this chain and in each case cytochrome molecules are reduced.
7. ATP is produced by electron transport. Three molecules of ATP are produced when NADH + H⁺ is oxidized and two molecules of ATP are produced when FADH₂ is oxidized (2) & (5).
Coenzymes of ETC
The creation of the electron transport chain occurs by some coenzymes. The coenzymes are as follows.
1. Nicotinamide adenine dinucleotide
2. Flavin mononucleotide
3. Flavin adenine dinucleotide
4. Co-Q (Coenzyme-Q)
5. – b
6. Cyt- c₁
7. Cyt- c
8. Cyt- a
9. Cyt- a₃ (3).
Location
The transport electron chain is the third stage of cellular respiration. This chain or system consists of a series of proteins containing oxidation-reduction groups. The next step in the glycolysis and Krebs cycle is the electron transport chain. The citric acid cycle completes the mitochondria of eukaryotic cells. After this cycle is completed, high-energy electrons are present in the mitochondria. The mitochondria are a cell organelle with a double membrane. Mitochondria consists of five parts. These are the outer membrane, the inner membrane, the intermembrane space, the cristae, and the matrix. The chain is located in the inner membrane of the mitochondria (1) & (2).
Components of the electron transport chain
There are five components or carriers that participate in the electron transport chain. All electrons are transferred by these carriers. These are-
1. Nicotinamide nucleotides
2. Cytochromes
3. Iron-sulfur proteins
4. Flavoproteins
5. Coenzyme Q (1).
Electron Transport Chain equation
The electron transport chain is a process of oxidation-reduction reactions. The equation of the electron transport chain is-
NADH + 1/ 2O₂ + H⁺ + ADP + Pi → NAD⁺ + ATP + H₂O (3).
Complex of the electron transport chain
The electron transport chain is a process or system of molecules that accept or reduce electrons easily. When hydrogen ions are moved through the protein and go down the electron transport chain, ATP is formed. This chain is located in the inner membrane of mitochondria. There are 5 complexes in this chain. Four of the complexes are the parts of this chain and complex v is concerned with the synthesis of ATP. There are also two mobile electron carriers. These are ubiquinone (UQ) and cytochrome- c. UQ acts as a mobile electron carrier between complex I and complex III and between complex II and III. The following is a discussion of these complexes (1).
1. Complex- I or NADH/ CoQ reductase
This complex contains flavoproteins (FMN) and 5 to 6 iron-sulfur (Fe-S) proteins. These are FeSN 1a, FeSN 1b, Fe-S N3, FeSN5, and Fe-SN2. N-symbolizes the NADH system. FeSN 1a, FeSN 1b, Fe-S N3, FeSN5, and Fe-SN2 are nonheme iron with sulfur molecules (2).
2. Complex- II or succinate-CoQ reductase
The complex- II consists of two polypeptides, succinate dehydrogenase, and Fe-S proteins, Fe-SS1, Fe-SS2, Fe-SS3. Here S- symbolizes the succinate system. Enzyme succinate dehydrogenase is present on the M-side of the inner membrane. Fe-SS3 is in the inner space of the inner membrane (5).
3. CoQ mobile carrier
It is the first mobile electron carrier of the electron transport chain. The CoQ or UQ acts as a mobile carrier between complex I and complex III and between complex-II and III. CoQ takes electrons from FMNH₂ of NADH dehydrogenase and FADH₂ of succinate dehydrogenase. This mobile electron carrier is located between the middle parts of the inner membrane of mitochondria (4).
4. Complex- III
Two cytochrome b, one Fe-S protein, and one cytochrome c₁ are located in the complex- III. There are an antimycin binding protein and two different core proteins. The two b-type cytochromes are bт (Cyt-b₅₆₅) and bк (cyt-b₅₆₀). The T and K symbolize Transducing and Keilin. Cytochrome b is located in the middle part between the lipid bilayers. Cytochrome c₁ is located on the C- side of the inner membrane (2).
5. Cytochrome c mobile carrier
It is also a mobile electron carrier. This carrier remains bound to complex-IV and transfers electrons from complex- III to complex- IV. The Cytochrome- c is the second mobile electron carrier (1) & (2).
6. Complex IV or cytochrome c oxidase
This complex is exposed at the both M-side and C-side of the inner membrane of the mitochondria. Cyt- a, Cyt- a₃ are located in this complex. Cyt- a is located on the C-side and Cyt- a₃ is located on the M-side. There are two copper centers are located between Cyt- a₃ and Cyt- a in the forms of Cu⁺⁺/ Cu⁺ redox pair. The complex- IV is an integral protein containing two heme and two copper centers. The two copper centers are Cu-alpha and Cu-beta (1) & (2).
Steps
1st Step
In the first step, Flavin mononucleotide (FMN) is reduced to FMNH₂ by taking hydrogen from NADH₂, and NADH₂ is oxidized by itself. One molecule of ATP is synthesized from ADP and inorganic phosphate (Pi) by using the energy released in this step.
2nd Step
A pair of hydrogen molecules from succinic acid is first transferred to FAD to form FADH₂.
3rd Step
The FMNH₂ and FADH₂ produced in the above two processes are oxidized by transferring their hydrogen to the coenzyme Q and Co-Q gets reduced to Co-QH₂.
4th Step
In the 4th stage, the Co-QH₂ reduces hydrogen and then converts it into electrons and protons.
2H → 2H²⁺ + 2e⁻
5th Step
In this stage of the electron transport chain, the oxidation of reduced Co-Q occurs by the transfer of electrons to cytochrome. At first, reduced Co-Q transfer electrons to cyt- b. it later decreases on its own. Now, Cyt-c₁ Cyt- a, Cyt- c, and Cyt- a₃ can be gradually reduced and oxidized by electrons.
6th Step
In the sixth or last stage of this system, Cyt- a₃ which is reduced in 5th stage loses a pair of electrons. A pair of electrons are accepted by molecular oxygen along with a pair of protons. As a result of these reactions, one molecule of water is formed. Here oxygen plays a vital role. Oxygen is the terminal acceptor of electrons in this chain.
1/2 O₂ + 2e⁻ → O⁻ ⁻ 2H⁺O⁻ ⁻ → H₂O
In this process or system, one molecule of ATP is formed between NAD and FMN. This ATP is the first molecule. Then the second and third molecules of ATP are produced. This process occurs when electrons are shifted from Cyt- b to Cyt- c₁ and Cyt- a to Cyt- a₃. The succinic gives its H₂ to FAD. ATP is not formed in this reaction. So 2 molecules of ATP are produced from each FADH₂ and 3 molecules of ATP are produced from each NADH + H⁺ (2) & (3).
Q&A
1. Where does the electron transport chain take place?
Mitochondria are the main place where the electron transport chain takes place. This chain or system takes place in the inner membrane of the mitochondria. The inner and outer membrane space of the mitochondria is called intermembrane space.
2. Where are the proteins of the electron transport chain located?
There are 5 protein complexes in this chain. These proteins are located in the inner membrane of the mitochondria.
3. Where does the electron transport chain occur?
The mitochondria of the eukaryotic cell consist of five parts. These are the outer membrane, inner membrane, intermembrane space, matrix, and cristae. The electron transport chain occurs in the inner mitochondrial membrane of the eukaryotic cells. The intermembrane space is located between the inner membrane and outer membrane of the mitochondria of the eukaryotic cell. In a prokaryotic cell, the chain occurs in the plasma membrane.
4. Where is the electron transport chain located?
The electron transport chain is located in the mitochondria of the eukaryotic cells and in the plasma membrane of prokaryotic cells.
References
1. B. Powar and G. R. Chatwal. Biochemistry, B. SC (general & honors course) and M. Sc. Himalaya publishing house, Chapter: Metabolism of carbohydrates. Page no- 550 to 559.
2. Ajoy Paul. Zoology Honours, Volume- 2, Books & Allied (P) Ltd. Chapter: Carbohydrates metabolism. Page no- 342 to 345
3. B Agarwal and V. K. Agarwal. Unified Botany, B.Sc. second Year. Shiva Lal Agarwal & Company Publications, Indore. Chapter: Respiration. Page no-158 to 159.
