Introduction
In a simple line, we can say that “X-ray crystallography is used to determine any solid compound’s molecular structure.”
Definition
X-ray crystallography is an analytical method based on the diffraction of X-rays by a crystalline material. This is the only technique that can provide the molecular structure of a given compound in a solid crystalline state.
- The discovery of X-rays was introduced by Wilhelm Röntgen in 1895.
- They were able to determine that each crystal lattice plane produces its unique diffraction pattern due to its specific arrangement of atoms.
Principle
The basic science of X-ray crystallography is based on the Principle of diffraction of X-rays. When a beam of X-rays passes through a crystal, it interacts with the electrons within the atoms of the crystal lattice.
This results in diffraction patterns that can be studied to determine the arrangement of atoms within the crystal.
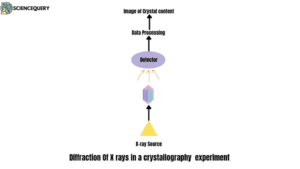
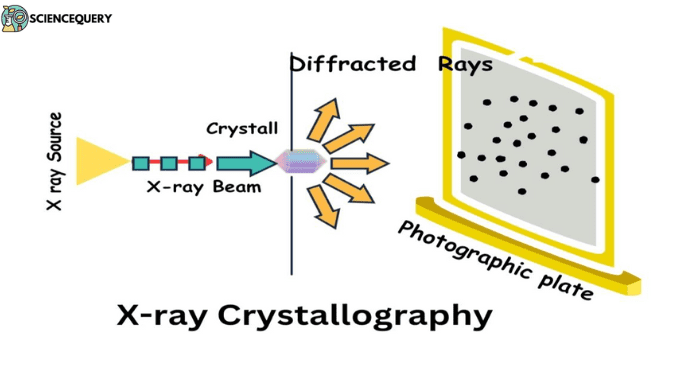
- To examine a crystal lattice, an X-ray diffraction technique is being used by the scientists.
- The crystal sample blocks the flow of X-rays from the source which results in scattering of rays.
- This form of scattering is known as diffraction.
The diffracted rays are then detected with the help of a detector. After the X-ray diffraction is collected by the detector, the data is processed and hence this process is termed as data processing. This process concludes with the generation of visual images (atoms, ions, or molecules) of the crystal lattices.
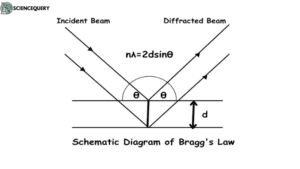
Bragg’s Law
Bragg’s law states that diffraction spots occur when 2dsin=nλ. In an X-ray diffraction experiment, the crystal diffracts the rays as per Bragg’s law.
Also, Miller’s indices are used to provide a visual representation of the planes in a crystal lattice. These indices are determined from the intercepts of the planes along the crystallographic axis.
- The smaller a Miller index is, the more parallel the plane is to the axis.
- The larger a miller index is, the more perpendicular the plane to the axis.
- If the Miller index is zero, the plane is parallel to the axis.
Description
- X-ray crystallography is a solid-state technique that provides very detailed and accurate information regarding a particular crystal study.
- This technique relies on the method of X-ray diffraction from crystalline specimens of varying sizes.
- This crystallography technique is generally divided into small molecular crystallography and macromolecular crystallography techniques.
Small Molecular Crystallography Techniques
It consists of organometallic molecules with sizes ranging from a few atoms to several hundred molecules. This method is commonly used in the identification and structural confirmation of newly synthesized drug molecules.
Macromolecular Crystallography
It is generally referred to as protein crystallography is used to study the three-dimensional structure of large biological molecules. For example nucleic acids, DNA, etc. This technique is used by scientists to study the secondary structure of the protein.
Applications
- X-ray crystallography plays a key role in drug design.
- In the characterization of a particular material.
- Determination of protein structure.
- Identification of minerals.
Advantages
- High resolution: X-ray crystallography provides minute details of molecular structures, thus allowing for precise analysis.
- Structure study: It is a solid-state technique used in the field of structural biology to determine the 3D structure of molecules.
- Cost-effective- X-Ray crystallography is relatively less expensive and requires minimal handling costs.
Disadvantages
- Difficulty with large molecules: X-rays have difficulty penetrating larger molecules such as proteins and nucleic acids.
- Radiation damage: The excessive radiation used in x-ray crystallography causes extreme damage to the biological molecules. It also leads to inaccurate results.
Q&A
1. What is X-ray crystallography and how does it work?
X-ray crystallography is an analytical method that is used to determine the atomic and molecular structure of crystals. It works by presenting a crystal specimen to an X-ray beam, which causes the crystal to undergo diffraction. Results in the scattering of the X-rays creating patterns.
Is simple process that begins with the selection of a pure crystal sample to be studied. It is placed on a specialized instrument called an X-ray diffractometer that emits an intense beam of X-rays onto the sample. An X-ray diffractometer is a specialized measurement device that helps in the proper analysis of crystals in solid or powdered form. This instrument works under the principle of Bragg’s law.
As these rays pass through the crystal, they interact with the electrons that vibrate at certain frequencies. These vibrations create diffraction patterns that are distinct for each type of atom present in the crystal. These patterns are captured by detectors and 3D images of atoms can be observed within the crystal lattice.
2. What is X-ray crystallography in genomics?
X-ray crystallography is more accessible and efficient, leading to its widespread use in various fields such as drug discovery, protein engineering, and structural biology.
3. What are the three components of X-ray crystallography?
Three components include:
- X-rays: These are high-energy electromagnetic waves that can penetrate through materials and interact with the atoms in a crystal lattice.
- Diffraction patterns: When an X-ray beam passes through a crystal, it gets scattered, thus producing a unique pattern of spots on photographic film.
- Analysis of pattern: Once data is obtained, mathematical calculations are performed using specialized software to reconstruct its 3D structure.
4. What are the advantages of X-ray crystallography?
With three-dimensional images, researchers are able to determine important information. They are able to analyze the chemical bonds present between atoms, and the distances between neighboring molecules. Hence, they can determine the overall structural features such as symmetry and packing arrangements.
Its ability to reveal detailed structures at atomic levels makes it one of the most powerful tools available for studying matter.
Summary
- X-ray crystallography is an analytical method that is based on the diffraction of X-rays.
- The discovery of X-rays was introduced by Wilhelm Röntgen in 1895. They were able to determine that each crystal lattice plane produces its own unique diffraction pattern due to its specific arrangement of atoms.
- The crystal sample blocks the flow of X-rays from the source which results in the scattering of rays. This form of scattering is known as diffraction.
- X-Ray crystallography is relatively less expensive and requires minimal handling costs.
- An X-ray diffractometer is a specialized measurement device that helps in the proper analysis of crystals in solid or powdered form. This instrument works under the principle of Bragg’s law.
- The vibrations create diffraction patterns that are distinct for each type of atom present in the crystal. These patterns are captured by detectors and 3D images of atoms can be observed within the crystal lattice.
- X-ray crystallography provides minute details of molecular structures, thus allowing for precise analysis.
References
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1186895/
https://kms.shanghaitech.edu.cn/bitstream/2MSLDSTB/1134/1/10.1002%40prot.20280.pdf
https://www.pnas.org/doi/abs/10.1073/pnas.261549098
https://pubs.acs.org/doi/abs/10.1021/acs.accounts.7b00366
https://www.mdpi.com/1420-3049/25/5/1030