Know in one minute about acidic amino acids
|
Introduction
Acidic amino acids are those amino acids that are slightly negative in charge. Before going through the topic let us first explore what are amino acids and how they are divided into positive and negatively charged amino acids.
What are amino acids?
Amino acids are organic molecules having two functional groups namely, the amino group (- NH2) and the carboxyl group (- COOH). Besides the amino group and a carboxyl group, it also has an alkyl group (R). Amino acids that have negatively charged groups in their side chain are called acidic amino acids.
Proteins are made up of amino acids. There are 20 amino acids that are required for protein synthesis. Amino acids differ from each other in their side chains or R-groups, attached to the α-carbon.
Classification of amino acids
On the basis of polarity, amino are classified into two groups
a. Non-polar or Hydrophobic
Mostly hydrocarbons are found in this group. These are divided into two subgroups
- Aliphatic side chain:- Aliphatic amino acids are both closed or open side chains and contain only hydrogen and carbon.
Examples are Alanine, valine, leucine, and Isoleucine.
- Aromatic side chain:- These amino acids contain aromatic rings or functional groups.
Examples are Tyrosine, Tryptophan, and phenylalanine.
b. Polar or hydrophilic
This group is further classified into three groups
- Acidic or negatively charged amino acids:– Those amino acids have a carboxyl group (- COOH) attached to their side chain. Glutamate and Aspartate are examples.
- Basic or positively charged amino acids:- Amino group (- NH2) group attached to their side chain. Examples are Arginine, Histidine, and Lysine.
- Neutral or Uncharged Amino Acids:- Amide group (- CONH2), alcohol group (-OH), and cysteine attached on their side chain. For example Glycine, serine, and Threonine.
Acidic amino acids and their examples
Aspartic acid and glutamic acid are examples of acidic amino acids.
- Both amino acids are non-essential amino acids. Non-essential means these amino acid bodies synthesize themselves, we don’t need to take them in our diet.
- And are glucogenic amino acids. That means they formed glucose after their complete degradation.
- These amino acids contain charged groups in their side chain so both are polar and hydrophilic acids. In hydrophilic amino acids, the side chains have a high affinity for water.
Characteristics of Aspartic acid
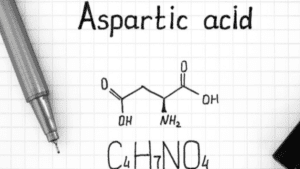
- Also known as aspartate. The three-letter abbreviation is Asp and the one-letter abbreviation is D.
- It contains carboxylic acid (- COOH), an amino group (-NH2), and hydrogen (- H), and the side chain will be a CH2 – COOH group.
- The carboxyl (- COOH) group of an amino acid can donate a proton (H+) and behave as an acid, forming a negatively charged anion.
- There are two negatively charged group present. That means there are more negatively charged groups present in comparison to positively charged groups.
- Asparagine is the amide form of Aspartate. The COOH group of dicarboxylic amino acids can combine with ammonia to form the corresponding amide.
Clinical aspects of asparagine
Asparginase worked as an antitumor agent. Tumour cells, mainly leukemic cells, require asparagine.
Synthesis of Aspartic Acid
Aspartic Acid is synthesized from oxaloacetate by transaminase reaction. Oxaloacetate is an intermediate of TCA.
Oxaloacetate + L Glutamate ⇔ Aspartate
Characteristics of Glutamic acid
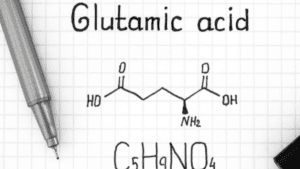
- Also known as glutamate.
- The three-letter abbreviation is Glu and one letter abbreviation is E.
- It contains carboxylic acid (- COOH), an amino group (- NH2), and hydrogen (-H) and the side chain will be CH2 – CH2 – COOH group.
- The amino group (-NH2) of an amino acid can accept the proton (H+) which behaves as a base, forming a positively charged cation.
- There are also two more negatively charged groups present in comparison to positively charged groups.
- Glutamine is an amide form of glutamate.
- Glutamine is reconverted to glutamate by a different enzyme, glutaminase, which is particularly important in the kidney.
Synthesis of Glutamic acid
- It is synthesized from alpha-ketoglutarate, an intermediate of the citric acid cycle, by transamination.
Aspartate + alpha-ketoglutarate ⇔ Glutamate
- This reaction is a reversible reaction. It is catalyzed by Aspartate transaminase (AST, SGOT) and pyridoxal Phosphate (PLP) involved as a coenzyme.
- By reductive amination of alpha-ketoglutarate by NH4+ catalyzed by glutamate dehydrogenase.
- This is a reversible reaction and the water molecule is expelled.
Alpha-ketoglutarate + NH4 ⇔ glutamate
How are acidic amino acids different from normal amino acids?
- The acidic behavior of acidic amino acids makes them different from normal amino acids.
- Acidic amino acids are non-essential amino acids but other normal amino acids are semi-essential or essential.
- Acidic amino acids have more than negatively charged groups in comparison to positively charged groups but other normal amino acids have a neutral number of positive and negative groups.
Structure of acidic amino acids
Glutamate and Aspartate are the two acidic amino acids. They contain mainly ammonia (NH4+), Hydrogen, carboxylic group (COOH–), and R group or side chain.
Structure of Aspartate
Three-letter abbreviation:- Asp
One letter abbreviation:- D
IUPAC Name- 2,amino, butane,1,4 dioic acid
- Aspartate is the most acidic amino acid. This amino acid is made with hydrogen, 1 ammonia group (- NH+2), a carboxylic group (COOH–)
- , and in the side chain CH2 – COOH group is attached.
- The properties of the side chain determine the property of amino acids. In this amino acid, the number of carboxylic acids is more than the amino group that’s why its nature is acidic.
Structure of Glutamic acid or glutamate
Three-word abbreviation:- Glu
One-word abbreviation:- E
IUPAC Name of glutamate:- 2,amino pantone, 1,5- dioic acid
- Glutamate contains a carboxyl group (COOH–), ammonia group (-NH+2), and hydrogen, and in the side chain CH2 – CH2 – COOH group is attached.
Difference between acidic and basic amino acid
Key points |
Acidic amino acids |
Basic amino acids |
| Definition | Are acidic in solution and are mono-amino dicarboxylic acids. | These are basic in solution and are diamino–monocarboxylic acids. |
| Examples | Glutamate (Glu), Aspartate (Asp) | Arginine (Arg), Histidine (His), Lysine (Lys) |
| Composition | Contains more than the number of carboxylic (COOH–) groups or negatively charged groups in comparison to positively charged groups. | Basic amino acids contain more than the number of ammonia (- NH+4) groups or positively charged groups in comparison to negatively charged groups. |
| pKa value | Acidic amino acids have low pKa values.
A low pKa value shows that the molecule is a strong acid and a high pKa value denotes the alkalinity of molecules. |
Basic amino acids have a high pKa value. |
| Synthesis | Formed from TCA cycle intermediates. | Are formed from acidic amino acids. Arginine and histidine are synthesized from Glutamate which is an acidic amino acid. |
| Esseintial/ non-esseintial | Acidic amino acids are non-essential | Basic amino acids are semi-essential except lysine which is a nutritionally essential amino acid. Semi-essential means they are partially synthesized in the human body.
|
| Glucogenic or Ketogenic character | Are glucogenic | Basic amino acids are Ketogenic. Ex. Lysine
|
| Hydrophilic positively or negatively charged amino acids
|
They are hydrophilic negatively charged | Are hydrophilic positively charged groups |
| Proton donation | The carboxylic group of acidic amino acids donate protons (H+) and act as acid. | Basic amino acids accept protons (H+) and behave as basic |
Uses of acidic amino acids
- Synthesis of essential amino acids
Arginine is an essential amino acid. It can be synthesized by aspartic amino acids.
In protein metabolism
Aspartic acid is essentially involved in Protein metabolism.
In the urea cycle, Aspartate reacts with citrulline to form argininosuccinate which is cleaved, forming an essential amino acid arginine and fumarate.
- Synthesis of Fumarate
Aspartic acid is involved in the synthesis of fumarate which is entered in the citric acid cycle.
- Formation of Adenosine monophosphate (AMP)
Aspartate reacts with inosine monophosphate (IMP) and produces AMP.
- Formation of glutathione
Glutamate is responsible for glutathione formation. It is one of the amino acid present in glutathione.
It is a type of antioxidant. Contains three amino acids namely glutamate, cysteine, and glycine.
- Synthesis of GABA ( gamma amino butyric acid)
Produce a neurotransmitter called GABA. Which is synthesized from the decarboxylation of Glutamic acid.
- Conversion to amide form of amino acids:-
Glutamate will be converted to glutamine, the amide form of amino acid. Glutamine is the major transport form of ammonia.
- Synthesis of other amino acids
Glutamate is required for the synthesis of proline.
Arginine is a basic amino acid, synthesized from glutamate.
- Formation of biologically important compounds
Glutamate and Aspartate formed pyrimidine bases.
Q&A
1. How many amino acids?
20 types of natural amino acids are found. Some of which are synthesized by the body itself and some we get through food.
2. Where are amino acids found? Where are amino acids stored when amino acids are degraded for energy?
Amino acids are the monomer units of protein. They are stored in the liver where they degrade for energy.
3. Where are amino acids located?
Amino acids are located in the liver after absorption from where they are used as per requirement.
4. How much amino acid is in the egg?
Egg contains all essential 11 amino acids. Phenylalanine, Methionine, Valine, Histidine, Threonine Arginine, Tryptophan, Lysine, Isoleucine, and Leucine are the essential amino acids.
Written By: Richa Pachori
References
Essentials of Biochemistry, Pankaja Naik
Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
