Introduction
To obtain an absorption spectrum, it is necessary to measure the absorbance of a substance. The instruments that are used to study the emission of electromagnetic radiation as a function of wavelength are called spectrophotometers. The samples that are studied in the ultraviolet region are usually gasses and hence put in the cells called cuvettes.
Principle
The basic principles of the construction of spectrophotometers include:
-
Stable and cheap radiant energy source
Materials that can be excited to high energy states by an electrical discharge or heating serve as excellent sources. The most commonly used sources of UV radiation are hydrogen lamps and deuterium lamps.
They consist of a pair of electrodes enclosed in a glass tube, along with a quartz window. This glass tube is filled with hydrogen or deuterium gas at low pressure. When a stabilized voltage is applied, they emit radiation ranging from 180 and 350 nm. Xenon lamps are highly used for ultraviolet radiation but the results are not as stable as hydrogen lamps.
-
Filters
Filters operate by absorbing light in all other regions. Filters work by resolving polychromatic light into a wide bandwidth of 40 nm and are used in colorimeters. One limitation of filters is that they have low transmittance capacity.
Monochromator resolves polychromatic radiation into its wavelengths and isolates these into narrow bands. The main components of a monochromator includes:
- An entrance slit- It allows the polychromatic light from the source.
- A collimating device- It enables the collimation of polychromatic light onto the dispersion device.
- A wavelength resolving device- It consists of a resolving device like prism or grating that breaks the radiation into component wavelengths.
- A focussing lens or mirror
- An exit slit- It allows the monochromatic light to escape.
-
Prisms
Simple glass prisms are used for visible range whereas silica or quartz prisms are used for ultraviolet region spectroscopy. Fluorite is used in vacuum ultraviolet range.
-
Gratings
These are often used in the monochromators of spectrophotometers operating in ultraviolet regions. The principle behind the dispersion of radiation by a grating is that it resolves light into its component wavelength. One of the main advantages of gratings is that it has higher resolving power than prisms.
-
Sample Containers
Samples when studied in the ultraviolet region are mainly gases or solutions and are put in cells called cuvettes. Quartz or fused silica cells are used in this UV region. Standard path length of these cuvettes are usually of 1cm.
-
Solvents
The solvents used in these regions include water, methyl-, ethyl-, isopropyl-alcohols, chloroform, hexane etc.
-
Detection Devices
There are three basic kinds of detectors while detection in the ultraviolet region. Photocells, photovoltaic cells and photomultiplier tubes.
-
Photovoltaic cells
They are also known as barrier layer cells. It includes semiconductor cells that are crystalline structures. A typical photocell consists of a thin coating of selenium over a thin transparent silver film. These cells have a long life and are inexpensive.
-
Phototubes
They are also known as photoemissive tubes whose currents are quite small and require amplification.
-
Photomultipliers
These detectors are designed to amplify the initial photoelectric effect.It can be used at very low light intensities. At higher intensities, they are unstable and are used widely in spectrophotometry. Photomultipliers are used in UV-vis spectrophotometry.
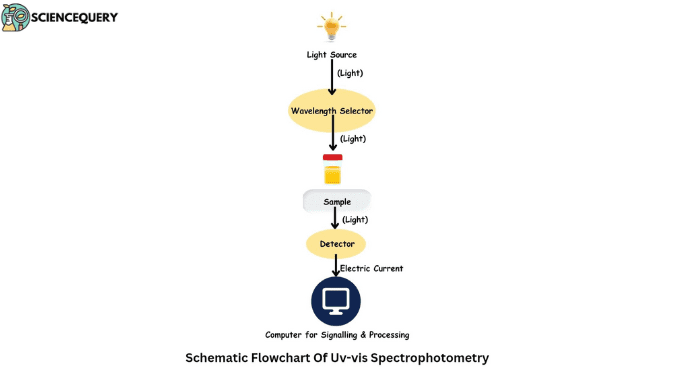
Method or how it works
UV-Vis spectrophotometry is an analytical technique that helps in measuring the absorption of light in the ultraviolet regions. This method works by passing a beam of light through a sample. Then it is measured how much of that light is absorbed by the sample. The basic principle behind UV-Vis spectrophotometry is based on Beer-Lambert’s law.
A= εLC where, A= absorbance, L= Path length, ε= Molar extinction coefficient and C= Sample concentration
This law states that the amount of light absorbed is proportional to the thickness of the absorbing material. It is independent of the intensity of the incident light.
1. UV-Vis spectrophotometers work by producing polychromatic white light from sources like tungsten-halogen lamps or deuterium lamps.
2. The monochromator inside the instrument separates this white light into its different components. Hence, it produces narrow bands of different wavelengths.
3. These individual wavelengths are then passed through the sample cell containing solutions before reaching the detector.
4. Beer Lambert law thus helps in quantifying the known concentrations of the known compounds.
Applications
- Qualitative Analysis- Ultraviolet spectra are used to identify compounds in pure state and biological preparations.
- Quantitative Analysis- In determining an unknown concentration of a given species by absorption spectrometry. A suitable absorption band is selected within the absorption spectrum for quantitative measurements.
- Most of the organic compounds of biological interest are absorbed in the UV-visible range of this spectrum.
- Some important classes of biological compounds are measured semi-quantitatively using UV visible spectrophotometers. For example- Nucleic acids at 254 nm and proteins at 280 nm are the best examples.
Advantages
- High sensitivity: UV-Vis spectrophotometers are highly sensitive instruments. They are capable of detecting minimal changes in absorbance.
- Wide range of applications: UV-Vis spectroscopy can be applied to a wide range of materials, including liquids, solids, and gases.
- Cost-effective: UV-Vis spectrophotometry is relatively affordable compared to other analytical techniques like NMR or X-ray crystallography.
- Easy data interpretation: Modern UV-Vis spectrophotometers have user-friendly interfaces and are easy to operate.
Disadvantages
- Interference from impurities: Impurities present in the sample can interfere with the absorption spectrum and hence affect the accuracy of results.
- Narrow range: UV-Vis spectrophotometry is limited to detecting compounds that absorb light and can interfere with data.
Q&A
What does UV-Vis spectrophotometry do?
UV-Vis spectrophotometry is an analytical technique that helps in measuring the absorption of light in the ultraviolet regions.
What data does UV-Vis spectroscopy tell you?
The data provide valuable information about the chemical composition, concentration, and structure of a sample.
What does a UV-Vis spectrophotometer study?
It works by measuring the intensity of light absorbed at specific wavelengths. It also determines its concentration within a mixture or solution.
What is the principle of UV-Vis spectroscopy?
The basic principle behind UV-Vis spectrophotometry is based on Beer-Lambert’s law. It states that the amount of light absorbed is proportional to the thickness of the absorbing material. It is independent of the intensity of the incident light.
Summary
- UV-Vis spectrophotometry is an analytical technique that helps in measuring the absorption of light in the ultraviolet regions. This method works by passing a beam of light through a sample.
- It works by producing polychromatic white light from sources like tungsten-halogen lamps or deuterium lamps.
- The samples that are studied in the ultraviolet region are usually gasses and hence put in the cells called cuvettes. Xenon lamps are highly used for ultraviolet radiation but the results are not as stable as hydrogen lamps.
- UV-Vis spectrophotometers are highly sensitive instruments. They are capable of detecting minimal changes in absorbance.
- Most of the organic compounds of biological interest are absorbed in the UV-visible range of this spectrum.
- Beer Lambert’s law thus helps in quantifying the known concentrations of the known compounds.
References
- https://books.google.com/books?hl=en&lr=&id=6ejwCAAAQBAJ&oi=fnd&pg=PA1&dq=uv-vis+spectrometry+principle&ots=bQI_sr-TVM&sig=lct1R0o_WHNZrg036cjYAP2lkS8
- https://link.springer.com/chapter/10.1007/978-1-4939-4029-5_1
- https://www.sciencedirect.com/science/article/pii/S0263224118311904
- https://pubs.acs.org/doi/pdf/10.1021/ed039p333
- http://vfsilesieux.free.fr/1Seuro/BeerLaw.pdf
