Know in one minute about Succinate dehydrogenase
|
Introduction
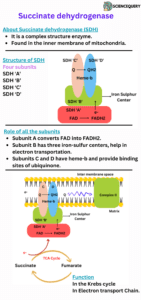
Succinate dehydrogenase is an enzyme found in the inner mitochondrial membrane. It is also a flavoprotein with FAD as the coenzyme. This can accept two hydrogen atoms (2H+or2e–) from Succinate. Also known as Succinate, ubiquinone oxidoreductase and complex II.
Reaction
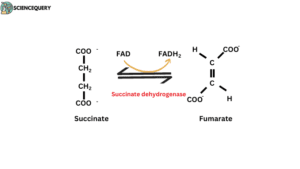
Succinate formed from succinyl CoA is Converted to fumarate in the presence of the Succinate dehydrogenase enzyme. This reaction is a part of the Krebs cycle which takes place in mitochondria.
Succinate + FAD ————–→ Fumarate + FADH2
Structure
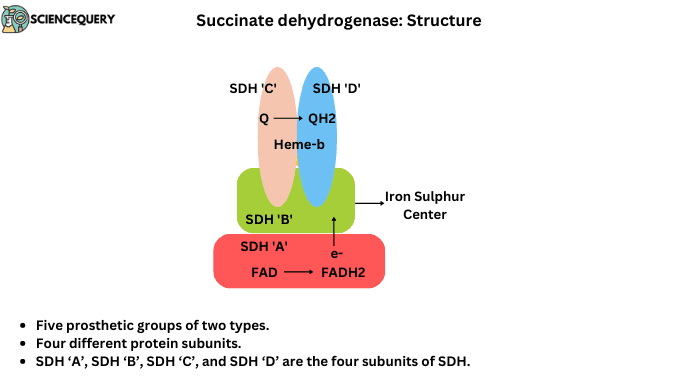
Succinate dehydrogenase (SDH) is a membrane-bound enzyme in the Krebs cycle, present in the inner mitochondrial membrane.
Succinate dehydrogenase contains five prosthetic groups of two types and four different protein subunits. SDH ‘A’, SDH ‘B’, SDH ‘C’, and SDH ‘D’ are the four subunits of SDH.
Subunit A
- It provides a catalytic site for succinate where succinate binds and converts to fumarate.
- In there FAD is found in covalently binding form. FAD convert to FADH2 from transfer Protons.
Subunit B
- This subunit is also called the iron sulphur centre containing the subunit.
- It contains three iron sulphur (Fe. S) centres.
- They are namely 2 iron 2 sulphur centre, four iron four sulphur centre, 3 iron four sulphur centre.
Subunits C and D
- They are integral membrane proteins, each With three transmembrane helices.
- They contain the heme group and binding site of Ubiquinone. Heme-b is covalently bound in this subunit.
- Heme- b is not directly involved in the electron transportation pathway. It’s reduced the frequency of electron licking.
Clinical significance of Heme-b
Leaked electrons formed reactive oxygen species (ROS) from combined with oxygen. Are directly bound with oxygen molecules and produce free radicals or ROS. These free radicals cause tissue damage.
If a mutation occurs in the surroundings of Heme- b and the binding site of ubiquinone. Heme-b is not capable of preventing electron leaking. As a result, free radicals are formed.
This type of mutation causes hereditary disease which is called paraganglioma. It is a type of cancer. In there, excessive amounts of free radicals or ROS are produced. They caused tissue damage and formed tumours.
The function of succinate dehydrogenase
Succinate dehydrogenase performs a dual function. It evolved in the kerb’s cycle and also participated in the electron transport chain.
1. In the Electron transport chain
In the electron transport chain, succinate dehydrogenase is known as complex II. It is involved in taking electrons from Succinate and passes to FAD followed by Fe-S centres and at the end ubiquinone.
2. In Krebs cycle
Acetyl CoA is fed into a cyclic pathway called the Krebs cycle. Krebs cycle is the second stage of cellular respiration. The succinate dehydrogenase enzyme worked as a catalyst in the Krebs cycle.
Succinate and fumarate are intermediates of this cycle. This is responsible for the conversion of Succinate to fumarate.
Succinate dehydrogenase mechanism
Electron transfer mechanism of succinate dehydrogenase
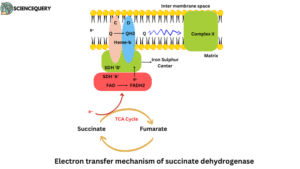
- In the electron transport chain, complex II is responsible for electron transportation. Have succinate dehydrogenase (type of flavoprotein) enzyme.
- Electrons pass from Succinate through the FAD and iron-sulfur centres or clusters before entering the chain of electron carriers in the mitochondrial inner membrane.
- Firstly the electron transfers substrates to FAD then FAD converts to FADH2.
- FADH2 transfers the two free electrons to the iron sulphur-containing centre.
- Three iron sulphur are present in subunit B. Electrons transfer continuously from one centre to the second centre and the second centre to the third iron sulphur centre.
- Electrons jump into the Ubiquinone from the third iron sulphur centre.
- Ubiquinone releases itself from subunits C and D. Ubiquinone is floating into the membrane and transferring the electrons into complex III.
- Complex III carries electrons from reduced ubiquinone to cytochrome c, and Complex IV completes the sequence by transferring electrons from cytochrome c to O2.
- The FAD to Ubiquinone distance is around 40Å. Which is a very large distance for electrons, they are not capable of travelling this distance. If electrons travel this distance they lose their energy.
- For this reason, the iron sulphur cluster braked this distance into smaller parts.
- They divide this distance around 11Å. This distance electrons easily travel and saves her energy.
- Electrons jumped from one iron sulphur cluster to the second iron cluster, in this type electrons moved ubiquinone. 1.5 ATP is produced per pair of electrons.
What is Ubiquinone?
Ubiquinone is known as Coenzyme-Q. It can accept electrons from FMNH2 produced in the ETC by NADH dehydrogenase or FADH2 produced Outside ETC (e.g. succinate dehydrogenase, acyl CoA dehydrogenase).
Mycobacteria do not have Complex IV.
Succinate dehydrogenase reaction
- Succinate dehydrogenase catalysed the Oxidation reaction in the Krebs cycle.
- The succinate formed from succinyl-CoA is oxidized to fumarate by the flavoprotein succinate dehydrogenase.
- Protons transfer Succinate to FAD, then FAD converts into FADH2. This reaction leads to the Oxidation of Succinate to fumarate.
Succinate dehydrogenase electron transport chain
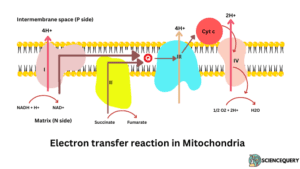
Electron transport chain
- NADH2 or FADH2 can not directly bind with oxygen. But through a chain of electron carrier chains which is called the electron transport chain.
- NADH2 or FADH2 formed in Krebs cycle. They are oxidized and released energy which is used for the synthesis of ATP
- Respiratory chains consist of 4 multienzyme complexes, namely Complex I, Complex II, complex III, complex IV
NADH dehydrogenase: Complex I
Succinate dehydrogenase: Complex II
Ubiquinol cytochrome reductase Complex III
Cytochrome oxidase – Complex IV
- Complexes I and II catalyze electron transfer to ubiquinone.
- Wherease, Complex II consists of the succinate dehydrogenase flavoprotein component. It is smaller and simpler than complex I.
- You can imagine it as a complex I without horizontal components.
- Complex II found in the inner membrane of mitochondria in eukaryotes and in prokaryotes is present in the cell membrane.
- Final electron acceptor in the reaction catalysed by Complex II. It is a complex structure, made with four subunits. They play an important role in electron transportation.
- Complex II transports two high-energy electrons to ubiquinone where they transfer to complex III.
Succinate dehydrogenase location
- Succinate dehydrogenase is one of the only membrane-bound enzymes of the Krebs cycle.
- Succinate dehydrogenase is a complex enzyme. It consists of four subunits. SDH ‘A’, SDH ‘B’, SDH ‘C’, SDH ‘D’.
- A and B subunits consist of bound FAD. 3Fe. S centre and Succinate binding site.
- C and D have bound ‘Heme- b’ and Q binding sites.
In eukaryotes, Succinate dehydrogenase is found in the inner membrane of mitochondria.
A and B extended matrix and C and D are the integral proteins having three transmembrane helices.
In prokaryotes, they are present in cell membranes. A and B subunits extended in the cytoplasm. As no mitochondria are there.
Q&A
1. Where is succinate dehydrogenase located?
It is located in the inner mitochondrial membrane.
2. What enzymatic reaction is catalysed by succinate dehydrogenase?
It worked as catalysed in the Krebs cycle. SDH converts Succinate to fumarate by transporting proton FAD to FADH2.
3. What is succinate dehydrogenase?
It is a complex structure enzyme found in the inner mitochondrial membrane. It plays an important role in the Krebs cycle and electron transfer chain.
4. What does succinate dehydrogenase do?
Converts succinate to Fumarate in the Krebs cycle and works as an electron transporter in the Krebs cycle as Complex II.
Written By: Richa Pachori
References
1. Biochemical Pathways, An Atlas of Biochemistry and molecular biology (edited by: General Michael)
2. Biochemistry 4th edition, (U. Satyanarayana U. Chakrapani)
3. Lehninger Principles of Biochemistry, 4th edition (David L. Nelson Michael M. Cox)
4. Clinical Biochemistry (William J. Marshall & Stephen K. Bangert)