Introduction
Protein is an essential part of our diet, consisting of long chains of amino acids called polypeptides. It plays a crucial role in building, maintaining, regulating, and repairing body tissue, and can be found in many different types of food. When we consume protein, our body breaks it down into individual amino acids during digestion. It is important to consume the right amount of protein to meet our body’s needs, as it helps repair and build muscle tissue. Every cell in the human body contains protein, making it an important nutrient for overall health and well-being. Let us now dive into the topic primary structure of proteins and their function.
Functions of Protein
It regulates many functions of our body. These are to produce Energy, hormone production, control enzyme activity, immunity developed, build Tissue and muscles, DNA replication, etc.
Amino acid
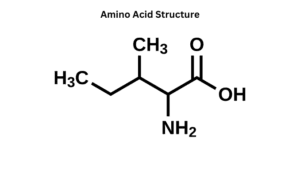
- Proteins are made up of amino acids. There are 20 amino acids that are required for protein synthesis.
- Amino acids are organic molecules having two functional groups namely, the amino group (-NH2) and the carboxyl group (-COOH).
- Besides the amino group and a carboxyl group, it also has an alkyl group (R).
- Each molecule of amino acid contains a central carbon atom known as an 𝛂-carbon. On this alpha carbon, both the amino and carboxylic groups are attached.
- The basic general formula is the same for all the types of amino acids; the only difference is because of the attached alkyl group.
Based on the types of side chains
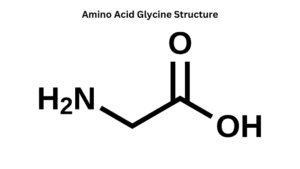
1. Hydrophobic (water-loving) amino acids: Examples of hydrophobic amino acids are glycine (Gly) and alanine (Ala).
2. Hydrophilic (water-repelling) amino acids: Examples of hydrophilic amino acids are serine and glutamine.
Based on polarity
1. Polar amino acids
This amino acid has a side chain that is capable of forming one or more hydrogen bonds. In a simple way, we call that polar amino acids are amino acids that have polarity. Examples of these amino acids are serine(Ser), asparagine(Asn), glutamine(Gln), threonine(Thr), cysteine(Cys), and tyrosine(Tyr). These amino acids have side chains that are polar but not charged.
2. Non-polar amino acids
This amino acid has the same numbers of the amine group and carboxylic group which makes this non-polar acid charge neutral. So these amino acids have no polarity
Examples of this amino acid are valine(Val), leucine(Leu), isoleucine(Ile), etc.
Based on their nutritional requirement
- Essential amino acids: Essential amino acids have to be taken in through food. 9 out of 20 amino acids are essential amino acids. Threonine. methionine, histidine, valine, leucine, phenylalanine, and tryptophan. lysine and isoleucine are essential amino acids.
- Non-essential amino acids. Nonessential amino acids have not to be taken in through food because they have been produced in our bodies. Aspartic amino acids, glutamic acid, tyrosine, serine, etc. are non-essential amino acids.
Protein structure and types
There are four levels of structure. These are the primary, secondary, tertiary, and quaternary structures of proteins. Here we discuss every structure and its examples.
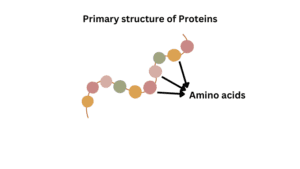
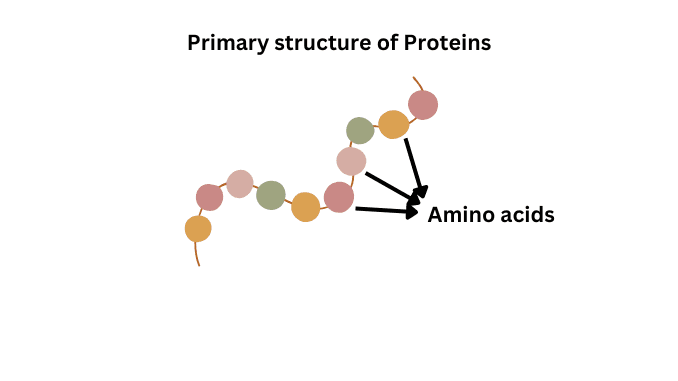
Primary structure
This is the first level of protein structure and it is the simplest structure. Amino acids in the polypeptide chain are held together by peptide bonds only. On the left side of the chain there is N-terminal amino acid and on the right side is C-terminal amino acid. In this structure, only covalent bonds are present like peptide bonds and disulfide bonds. Some examples of primary structural proteins are
1. Insulin
It is a hormone produced by the beta cells of the pancreatic islets. It controls blood sugar levels in our bodies. F.Sanger determined the sequence of amino acids in insulin. Insulin has 51 amino acids and it has two polypeptide chains, 𝛂 and 𝜷. 𝛂 chain has 21 amino acids and 𝜷 chain has 30 amino acids. Two polypeptide chains are connected by disulfide bonds.
2 Hemoglobin
It is the protein in the red blood cells of living cells. It carries oxygen from the lungs to cells in our body. Hemoglobin has 574 amino acids and four polypeptide chains. It has two alpha chains and two beta chains.
Each alpha chain has 141 amino acids and each beta chain has 146 amino acids. Sequences of amino acids in alpha chains are different from the beta chains. Every protein has specific arrangements of amino acids. Protein functions based on the sequence of amino acids.
Replacement of amino acids causes abnormal function of the protein. So, replace the amino acid position in the 𝜷 chain then hemoglobin becomes sickle because they do not carry oxygen in our body. This disease is called sickle cell hemoglobin or sickle cell anemia.
The main function of the primary structure of a protein is the intermolecular bonding of the amino acid chain. These structures provide a unique structure of a protein and determine the protein’s function.
Secondary structure
The polypeptide chain is linked by peptide bonds as well as hydrogen bonds. This structure is formed due to the formation of peptide bonds and intramolecular (within the molecule) and intermolecular (between two molecules) H-bonds due to the interaction between amino acids. These secondary structures are defined by patterns of the hydrogen bond. There are two structural models of this structure the 𝛂-helix and 𝜷-pleated sheets are shown by Linius Pauling and Michel Corey.
1. 𝛂-helix structure
In which polypeptide bond has spiral conformation. Each spiral turns has 3.6 amino acids. The amino acids come closer due to intramolecular H-bonding and Spiral held together by H-bond. It has normal geometry conformation. In this structure, the carbonyl (C=O) of one amino acid is hydrogen bonded to the amino (N-H) of an amino acid.
2. 𝜷-pleated sheets
It is a secondary protein structure in which polypeptide chains have backfolded. In this structure, two or more different polypeptide chains arrange next to each other and form a sheet plate-like structure held together by hydrogen bonds. Each polypeptide chain is called 𝜷-strand. This sheet is stabilized by hydrogen bonds between NH and CO groups in different polypeptide strands.
Examples
Hemoglobin, Keratin of hair, finger, and toenails, nucleic acid such as the cloverleaf structure of tRNA, etc are the best examples of this structure.
Tertiary structure
The term “tertiary structure” refers to the three-dimensional conformation that is driven by the interaction between R groups of polypeptides. The functional structure of this structure is globular protein. It is stabilized by H-bonds, hydrophobic bonds, hydrophilic bonds, electrostatic interactions, van der Waal’s forces, and electrostatic bonds. The function of a protein depends on this structure. if this structure is disordered then the protein loses its function. This structure can be studied by X-ray crystallography and Nuclear Magnetic Resonance studies.
Types of bonds stabilize a tertiary structure
Electrostatic interactions
These bonds are established between oppositely charged R groups of internal amino acid and charged 𝜶 groups of C and N terminal residues. The bonds of two opposite-charge ionic protein groups are called salt bridges.
Hydrogen bonds
This bond is the most important for the structure of the protein. It is also present in the secondary structure of the protein. The polypeptide chain is linked by peptide bonds as well as hydrogen bonds. This bond results from an attractive interaction between an electronegative atom and a hydrogen atom attached to a second electronegative atom. Oxygen and nitrogen are only two electronegative atoms, which participate in hydrogen bond formation. It is usually weaker on the protein surface area than the protein interior area because it is much more likely to complete interactions with water on the protein surface area. For example, DNA recognizes that hydrogen bonds between adenine-thymine base pairs and guanine-cytosine base pairs contribute to the stability of the helix structure.
Van der Waals force
This interaction is a weak electrostatic interaction between two polar groups, wo non-polar groups, or a polar group and a non-polar group.
Hydrophobic bond
These are formed due to non-polar side chains of the amino acids in the interior protein. When a nonpolar molecule or group is placed in water, water molecules interact through hydrogen bonds to form highly ordered cages around the non-polar molecules or group. These are weak interactions, therefore these are not true bonds. They contribute significantly to maintaining protein structure.
Example
All enzymatic proteins, plasma proteins like antibodies, myoglobin, etc.
Quaternary structure
In this structure, it will be formed of two or more two polypeptide chains which will be linked together by noncovalent interaction i.e hydrogen bond, hydrophobic bond, van der Waals force, etc. The polypeptide chains that are joined together, called subunits and proteins, will function only if all subunits are present properly. There can be multiple subunits and every subunit is sometimes associated with an organic ether non-organic component which is called the prosthetic group. A quaternary structure of a protein refers to the assembly of multiple folded molecules in a subunit complex. If there are only two subunits, the protein is said to be a dimer. If three subunits, the protein is called trimer, etc. This structure allows a protein to have multifunction. For example, one polypeptide chain may have a different orientation of conformation and it can perform one function.
Examples
Examples are DNA polymerase enzymes used for DNA replication and collagen protein which is found in connective tissues. hemoglobin which is used for oxygen transport, lactate dehydrogenase, an enzyme that is used in glycolysis, ribosomes, antibodies, and different ion channels.
Q&A
1. Which bonds are created during the formation of the primary structure of a protein?
Peptide bonds are created during the formation of the primary structure of a protein.
2. What is the primary structure of a protein?
This is the first level of protein structure and it is the simplest structure. Amino acids in the polypeptide chain are held together by peptide bonds only. These structures provide a unique structure of a protein and determine the protein’s function.
3. What determines the primary structure of a protein?
The amino acid sequence of this structure determines the primary structure of the protein.
4. Which of the following actions would affect the secondary, but not primary, structure of a protein?
Breaking the hydrogen bonds between amino acids because this bond makes a more stabilized secondary structure.