
Know in one minute about Primary battery
|
Introduction
A battery is a device or an arrangement that produces electrical energy through a set of chemical reactions. Any battery or cell that we use as a source of electrical energy is basically a galvanic cell where the chemical energy of the redox reaction is converted into electrical energy. There are two types of batteries – Primary and secondary batteries. In this article, we will discuss the primary battery in detail.
In primary batteries, the reaction occurs only once, and after use over a period of time battery becomes dead and cannot be reused again. The most familiar example of this type is the dry cell (known as Leclanche cell after its discoverer) which is commonly used in our transistors and clocks.
Primary battery: Structure or Diagram
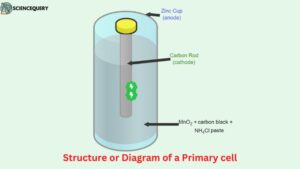
- As we can see in this illustration, the primary cell (specifically a dry cell) is made from components like an anode, cathode, and a solution which is in the form of a semi-solid paste.
- The container of this cell (Leclanche cell) is made from Zinc which also acts like an anode. While a rod is placed at the center of the cell which is made from carbon and acts like a cathode for the cell.
- Now since this kind of cell is known as a dry cell hence instead of an aqueous electrolyte a semi-solid electrolyte which is in the form of a paste is used to transfer or create a medium for the ions to easily transmit from one electrode to another.
Primary battery: Description
Now let us understand the working of a primary cell. Since leclanche cell is one of the popular primary cells used in day-to-day life hence we will understand the working primary cell with the help of leclanche cell.
The cell consists of a zinc container that reacts as an anode and the cathode is a carbon rod surrounded by powdered manganese dioxide and carbon.
The space between the electrodes is filled by a moist paste of ammonium chloride (NH4Cl) and zinc chloride (ZnCl2). The electrode reactions are complex, but they can be written approximately as follows :
At anode : Zn(s) → Zn2+ + 2e–
At cathode :
MnO2 + NH+4 + e– → MnO(OH) + NH3
In the reaction at the cathode, manganese is reduced from the +4 oxidation state to the +3 state. Ammonia is produced in the reaction and forms a complex with Zn2+ to give [Zn (NH3)4]2+. The cell has a potential of nearly 1.5 V.
Primary battery: Types
According to use and functionality, there are several types of primary cells currently available in the market.
- Alkaline batteries
- Mercury batteries
- Silver Oxide batteries
- Zinc-Air batteries
- Lithium batteries (not lithium-ion batteries)
1. Alkaline Batteries
Alkaline batteries are widely used in day-to-day life, almost all the batteries which are non-rechargeable like AAA or AA batteries are alkaline batteries.
The arrangement or the circuit of an alkaline battery is similar to that of a Leclanche cell but instead of a carbon rod, it uses manganese dioxide or nickel and cadmium or nickel and hydrogen. The container is made up of zinc and is also the anode of the cell.
It uses an alkaline electrolyte of KOH (potassium hydroxide) instead of acidic NH4Cl or Zinc chloride. Hence it is known as an alkaline battery.
Furthermore, it provides higher energy density with longer shelf life than a leclanche battery of the same voltage.
2. Mercury Battery
Mercury batteries generate electricity using the reaction between mercuric oxide and zinc electrodes. The overall reaction for the battery is
Zn + HgO → ZnO + Hg
These batteries were used for products that require low currents. For example hearing aids, calculators, and electronic watches.
Except for small-sized cells (button cells specifically), the use of mercury in making batteries was banned.
3. Silver Oxide batteries
These batteries have a high energy-to-weight ratio which makes them useful in supplying electricity to small-sized gadgets.
These cells can maintain a nearly constant voltage during discharge up till they are completely depleted.
It consists of a silver oxide cathode while zinc is the anode. Due to the use of silver oxide the cost of production of a silver oxide cell is very high. Hence, the amount of silver used has been restricted by the authorities.
Q&A
1. Which kind of cell or battery was used in the apollo mission and why?
Silver oxide batteries were used in the apollo mission lunar program for its lunar module and lunar rover. The reason for using silver oxide batteries was their high energy-to-weight ratio. These batteries can provide high energy while being light in weight.
2. What are primary batteries and why zinc is used in them?
Primary batteries are a non-rechargeable, portable man-made source of electricity. They provide electricity with the help of reactions between their anode and cathode elements.
Zinc is widely used in primary cells as it provides high energy density as compared to other elements.
Reference
https://ncert.nic.in/ncerts/l/lech103.pdf
Written By: Bharat Awasthi
