Know about Physisorption in one minute
|
Introduction
Knowing about the roots of a tree before examining it is always considered to be a good practice. So, before learning about physisorption, let us understand the term adsorption.
Adsorption is the phenomenon in which a substance is concentrated only at the surface and does not penetrate through the surface to the bulk of the adsorbent.
There are mainly two types of adsorption of gases on solids.
- Physisorption
- Chemisorption
Definition
Whenever gaseous particles are accumulated on the surface of a solid and there is a weak Van Der Waal’s force between the molecules of adsorbate and the surface molecules of the adsorbent then the phenomenon being held is known as Physisorption.
- Physical adsorption at low temperatures may pass into chemisorption as the temperature is increased. For example, dihydrogen is first adsorbed on nickel by van der Waal’s forces. Molecules of hydrogen then dissociate to form hydrogen atoms which are held on the surface by chemisorption.
- Unlike chemisorption which consists of chemical bond formation between the adsorbent surface and adsorbate, physisorption is a reversible process. And does not result in the formation of a new chemical species.
- Physisorption typically occurs at low temperatures and low pressures. And the strength of the interaction between the adsorbate and the adsorbent surface increases with decreasing temperature.
- Physisorption plays an important role in a wide range of applications, including gas storage, catalysis, and separation processes.
- The amount of gas or liquid that can be adsorbed by a given material through physisorption depends on factors. These are the surface area of the material, the nature of the adsorbate and the adsorbent surface, and the temperature and pressure of the system.
Characteristics
1. Lack of specificity
For a given surface of an adsorbent, there is no preference for a particular gas as van der Waal’s forces are universal.
2. Nature of adsorbate
The amount of gas adsorbed by a solid depends upon the nature of the gas. In general, easily liquefiable gases (i.e. with high critical temperature) are readily adsorbed as van der Waal’s forces are stronger near the critical temperatures.
Thus, 1g of activated charcoal adsorbs more sulfur dioxide (critical temperature 630K), than methane (critical temperature 190K) which is still more than 4.5 mL of dihydrogen (critical temperature 33K).
3. Reversible nature
Whenever physical adsorption occurs between a gas and a solid it is often reversible in nature. By Le-Chatelier’s principle, the amount of gas adsorbed increases when the pressure is increased this happens because the volume of the gas decreases. While the pressure is decreased the gas can be removed.
Also, the process of adsorption is exothermic in nature, the physical adsorption occurs readily at low temperatures and decreases with increasing temperatures.
4. Surface area of the adsorbent
As the surface area of the adsorbent increases the extent of adsorption increases. Finely divided metals and porous substances having large surface areas are good adsorbents.
5. Enthalpy of adsorption
Although physical adsorption is an exothermic process its enthalpy is quite low, approximately 20-40 kJ mol-1.
Diagrams And Equations
In order to study adsorption occurring between two substances an isotherm is taken into account.
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed an adsorption isotherm.
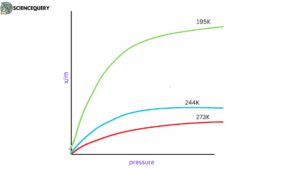
There are several types of isotherms, but 3 of them are the most popular
- Langmuir adsorption isotherm
- BET adsorption isotherm
- Freundlich adsorption isotherm
1. Langmuir adsorption isotherm
The Langmuir isotherm is a model that describes the monolayer adsorption of gas or liquid molecules onto a surface. It assumes that the adsorbent surface has a finite number of identical, non-interacting adsorption sites and that the adsorption process is reversible and follows first-order kinetics. The equation is:
θ = (k * P) / ( 1 + k * P)
Where θ is the fractional surface coverage, K is the Langmuir adsorption constant, and P is the pressure of the gas or liquid.
2. BET adsorption isotherm
The BET (Brunauer-Emmett-Teller) adsorption isotherm is a more general model that accounts for the formation of multiple layers of adsorbate molecules on the surface.
3. Freundlich adsorption isotherm:
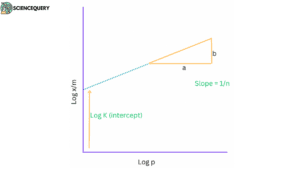
The Freundlich adsorption isotherm is another empirical model that describes physisorption. It assumes that the surface is heterogeneous and that the adsorption process follows a power-law dependence on the concentration of the adsorbate. The equation is:
q = k * c1/n
where q is the amount of adsorbate adsorbed per unit mass of adsorbent, C is the concentration of the adsorbate in the gas or liquid phase, K is the Freundlich constant, and n is a parameter related to the strength of the adsorbate-adsorbent interaction.
Some practice problems
1. Why does physisorption decrease with the increase in temperature?
Ans. Physisorption decreases with an increase in temperature, this happens because of Le Chatelier’s principle.
2. What is Freundlich Adsorption isotherm?
Ans. Freundlich, in 1909, gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature.
Q&A
1. What is physisorption?
Ans. Physisorption, also known as van der Waals adsorption or physical adsorption, is a type of adsorption process in which a gas or liquid molecule adheres to the surface of a solid material through weak van der Waals forces
2. What is the main difference between physisorption and chemisorption?
Ans. The main difference between physisorption and chemisorption is Physisorption arises due to weak van der Waal’s forces, while chemisorption is caused by the formation of chemical bonds. Physisorption is reversible while chemisorption is irreversible.
3. Why physisorption is reversible?
Ans. Physisorption is reversible as, when the pressure is decreased the gas molecules or the adsorbate can be removed.
4. What is molecular physisorption?
Ans. Molecular physisorption is a type of adsorption process in which molecules are attracted to a surface through weak van der Waals forces. These forces are temporary and arise from fluctuations in the electron density of the molecules and the surface.
Written By: Bharat Awasthi
