Introduction
Peptide bonds are the linkage or attachment of one amino group and one carboxyl group from amino acids. Amino acids are the building blocks of proteins. Protein is the main component of human body cells. These acids are obtained by splitting large protein molecules into smaller ones.
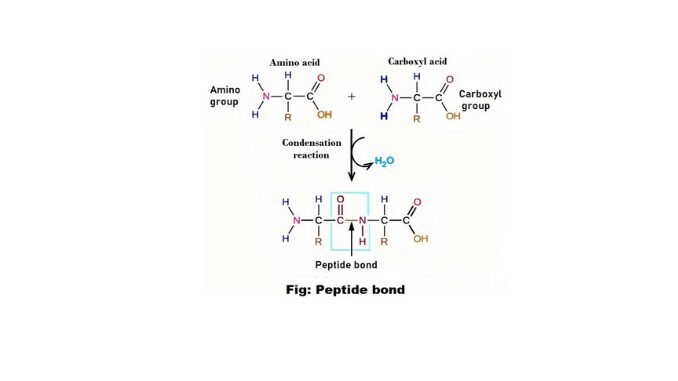
Amino acid is an essential component of protein. Each amino acid contains at least one amino group (-NH₂) and one carboxyl group (-COOH). The carboxyl group is acidic and the amino group is alkaline. The Amino group is usually attached to the carbon next to the carboxyl group. One amino group of amino acids and one carboxyl group are attached together and form a linkage. This linkage is known as peptide bonds (4).
Protein, peptides, and amino acids are interrelated but their properties are different. Peptides are made up of two or more amino acids, which are attached to peptide bonds. Thousands of amino acid molecules in a protein are joined together by this bond. So this bond is very important. Its structure and function are described in detail below (1) & (3).
Peptide bond
Emil Fischer first observed the peptide bonds. The polymers of amino acids are peptides. Large peptides are called polypeptides or proteins. Two amino acid molecules are covalently joined through a substitute amino linkage, termed a peptide bond. Such a linkage is formed by the removal of the elements of water from the α-carboxyl group of one amino acid and the α-amino group of another.
The peptide bond formation is a condensation reaction. Proteins are formed by the repetition of amino acids, linked by peptide bonds. Therefore any protein has –NH₂ and –COOH terminal groups. The former is N-terminal and the latter is C-terminal (4) & (2).
What is a peptide?
A peptide is a polymer of amino acids linked by amide bonds between the amino group of each amino acid and the carboxyl group of the neighboring amino acid. Mainly they are short chains of amino acids. Each amino acid unit in the peptide is known as the residue. It is formed by the peptide bond (1).
Types of peptide bond
There are various types of peptide bonds.
1. Dipeptide bond
If there are two amino acid residues, the peptide is called a dipeptide and the linkage is known as a dipeptide bond or linkage.
2. Tripeptide bond
When three amino acids are attached together, a linkage forms. This is a tripeptide bond.
3. Oligopeptide bond
If there are four to ten amino acid residues, the bond is called the Oligopeptide bond, and the peptide is known as the Oligopeptide.
4. Polypeptide bond
This bond is made up of more than 10 amino acid residues and less than 50 amino acid residues. If there are more than 50 amino acid residues, it is called a protein (4).
Some interesting features of peptide bonds
The amino acids have a carboxyl group and an amino group bonded to the same carbon atom. They are bound together to form peptide linkage or bond. Both groups differ from each other in their side chains or R groups. Properties of peptide bonds are
1. The peptide bond is a covalent bond formed between the α-amino group of one amino acid and the α-Carboxyl group of another amino acid.
2. These bonds are responsible for the formation of polymers called proteins.
3. The formation of a peptide bond occurs during protein synthesis with the help of ribosomes.
4. Peptide bonds help in the formation of polypeptide chain polymers.
5. It is a rigid and planar bond. So they can stabilize the protein.
6. This bond or linkage is the basis of most biological reactions.
7. The formation of peptide bonds takes up energy, which is derived from ATP in the organism.
8. All amino acid molecules are bound inside the protein by this bond.
9. This bond is broken by the proteolytic enzyme.
10. Peptide bonds cannot be destroyed by normal temperature. But if temperatures above 110°C are applied for 48 hours, it is possible to destroy this bond (4) & (1).
Structure of Peptide bonds
- A peptide bond is a type of covalent bond. This bond is formed between two amino acids. It is formed through a dehydration reaction.
- When hydrogen and oxygen are removed from the amino acid then this bond is formed.
- The bond also shows a double bond character. It is a chemical covalent bond formed between the alpha-amino group of one amino acid and the alpha-carboxyl group of another amino acid.
- Due to the closeness of the carbonyl carbon-oxygen double bond allowing the resonance structures to exist.
- Because of this, the carbon and nitrogen bond is also shorter than normal carbon-nitrogen single bonds. The peptide chain which is made up of the CO-NH atoms is thus relatively rigid and planar, although free rotation can take place about the bonds on either side of the peptide bond.
- Scientists Robert Corey and Linus Pauling first discovered that peptide bonds are rigid and planer.
The hydrogen of the amino group is nearly always on the opposite side (Trans) of the double bond to the oxygen of the carbonyl group, rather than on the same side (cis). The atoms of the peptide bond are in the same plane as the hydrogen of the alpha-amino group and the oxygen of the alpha-carboxyl group are Trans to each other (3).
The function of Peptide bonds
Peptide bonds participate in some functional activities.
1. It forms a large chain of amino acids, known as Proteins. Proteins are the building blocks of all living organisms.
2. The peptide molecules of this bond act as structural components of cells and tissues, hormones, toxins, antibiotics, and enzymes.
3. Each peptide bond can participate in two hydrogen bonds. In one, the –NH group acts as a hydrogen donor, and in the other, the –CO group acts as a hydrogen receptor.
4. A single peptide bond is an organism that binds the carboxyl group of amino acids to the amino group of alpha-carbon atoms of other amino acids.
5. Peptide molecules of peptide bonds can be biologically active on their own or they can act as a subunit for larger molecules.
6. It acts as a transporter. This bond allows molecules to pass through the cell membrane.
7. This bond or linkage attaches the –COOH group of one amino acid and the –NH₂ group of another amino acid and releases water molecules.
8. Peptide bonds link many amino acids together.
9. These bonds play an important role in muscle formation.
10. It plays an essential role as an anti-cancer agent.
11. The bond forms various types of peptides by the combination of different types of amino acids (4) & (2).
Q&A
1. What are peptide bonds?
The amino group of an amino acid molecule condenses with the carboxyl group of another to form an amide. The peptide bond is the amide linkage formed between the amino acids (3).
2. What is the name given to the reaction that breaks peptide bonds?
The hydrolysis process helps in breaking the peptide bond. A peptide molecule will break down in the presence of water and generate 2 to 4 kcal/mol of energy.
3. How are peptide bonds formed?
Each amino acid contains at least one amino group (-NH₂) and one carboxyl group (-COOH). The Amino group is usually attached with the carbon next to the carboxyl group. One amino group of amino acids and one carboxyl group are attached together and form peptide bonds.
4. Which of the following statements about peptide bonds are true?
Peptides are the polymers of amino acids that combine to form proteins. The bonds form from nucleophilic attack by an electron of an amino nitrogen atom of one amino group and a carboxyl carbon molecule of another amino acid.
5. Proteins are composed of chains which are joined together with peptide bonds?
Peptide bonds help in joining the amino acids together.
Written By: Manisha Bharati
References
1. B. Powar and G. R. Chatwal. Biochemistry, B. SC (general & honours course) and M. Sc. Himalaya publishing house, Chapter: Peptides and Proteins. Page no- 178 to 191.
2. Ajoy Paul. Zoology Honours, volume- 1, Books & Allied (P) Ltd. Chapter: Proteins. Page no- 771 to 792.
3. Nimai Tewari. Advance Organic Chemistry. Books and Allied (P) Ltd. Chapter: Amino acids, peptides, proteins enzymes and nucleic acids. Page No: 716 to 718.
