Introduction
There are various elements in nature and nitrogen is one of the most abundant elements. Nitrogen is a major component of protein which is the main building block for all organisms. And nitrogen is essential for the formation of cells in the body of an organism. Plants receive this nitrogen directly from the air whereas animals cannot take nitrogen directly from the air. So they are completely dependent on plants for nitrogenous substances. This nitrogen circulates in the physical and biological environment and forms the nitrogen cycle in nature. Nitrogen stores in nature are inexhaustible through the nitrogen cycle. The nitrogen cycle is completed via several steps. One such process in the nitrogen cycle is nitrogen (2) & (5).
Amount and form of nitrogen in the atmosphere
The primary source of nitrogen used in the nitrogen cycle is the atmosphere. This element is abundant in the atmosphere. The atmosphere contains 78 percent nitrogen out of 100 percent. Nitrogen exists in various compounds in the physical environment and biological environment.
In the gaseous cycle, nitrogen moves through the biosphere. In this case, the atmosphere, which is composed of about 78% nitrogen, acts as a reservoir for the organism (A. N. Strahler & A. H. Strahler 1976). Nitrogen is found in various forms in nature. Nitrogen is usually present in the atmosphere in some forms (1).
- Molecular nitrogen (N₂)
- Nitrous oxide (N₂O)
- Nitric oxide (No)
- Nitrogen peroxide (NO₂)
- Ammonia (NH₃)
- Nitrous acid (HNO₂)
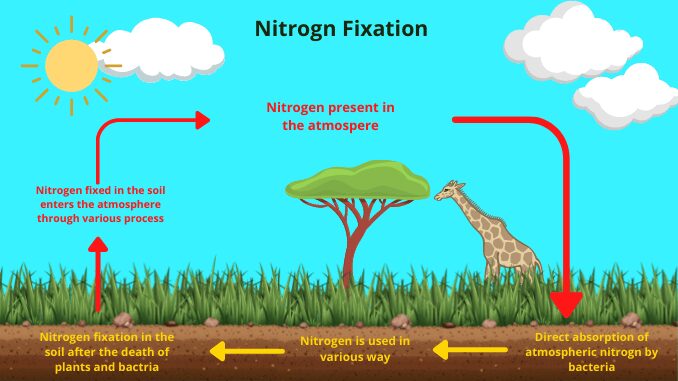
Nitrogen fixation
Nitrogen is present in the atmosphere in the form of di-nitrogen (N₂), which is inactive. Organisms cannot directly absorb this nitrogen from the atmosphere. So the element needs to be converted to take up nitrogen from the atmosphere. Nitrogen fixation is the process of nitrogen conversion. Nitrogen is absorbed by plants only when it is converted to ammonia and nitrate. And animals take nitrogen from plants. In this way, nitrogen is transferred from the plant’s body to animals through the food chain (1) & (3).
Definition
Nitrogen fixation is a process in which atmospheric nitrogen gas is converted to nitrogenous compounds and bounded or stored in the soil by various bacteria. Nitrogen fixation is the first step of the nitrogen cycle. In this process nitrogen in the atmosphere is converted into ammonia and nitrate. It is the process of converting an unusable form of nitrogen into a usable form (4).
Bacteria that help in nitrogen fixation
Nitrogen in the atmosphere remains non-reactive. So this element cannot be taken directly by animals or plants. Therefore, the conversion of the element (nitrogen) is required. But there are some bacteria in the environment that can absorb atmospheric nitrogen directly. They play an important role in nitrogen fixation. Bacteria that help in nitrogen fixation are Azotobacter, Bacillus, Clostridium, purple bacteria, Rhodospirillum, Pseudomonas, etc. (2).
| Free-living bacteria | Symbiotic bacteria | |
| Aerobic bacteria | Anaerobic bacteria | Rhizobium |
| Azospirillum | ||
| Azotobacter | Clostridium | |
| Klebsiella | Purple sulfur bacteria | |
| Cyanobacteria | Green sulfur bacteria | |
Process of Nitrogen fixation
The nitrogen present in the atmosphere circulates from the physical environment to the biological environment and again from the biological environment to the physical environment to form the nitrogen cycle. Nitrogen fixation is the first step of the nitrogen cycle. It is the process by which nitrogen present in the earth’s atmosphere is converted to ammonia (NH₃) or other molecules available for living organisms. Nitrogen fixation is achieved in two ways in nature (3).
Physical process
- The interaction of nitrogen, hydrogen, and oxygen in the atmosphere requires a lot of energy. The main source of this energy is cosmic radiation, the inherent energy of meteorites, lightning, etc. During lightning strikes in the sky, atmospheric nitrogen combines with oxygen to form nitric oxide.
N₂ (Nitrogen) + O₂ (Oxygen) → 2NO (Nitric oxide)
- In the second stage, the nitric oxide reacts with oxygen in the air to form nitrogen peroxide.
2NO (Nitric oxide) + O₂ (Oxygen) → 2NO₂ (Nitrogen peroxide)
- In the third stage, the nitrogen peroxide reacts with rainwater to form nitrous and nitric acid. Rainwater carries this acid to the soil.
2NO + H₂O + O₂ → HNO₂ + HNO₃
- In the fourth stage, these two types of acids combine with the salts of calcium, potassium, etc. in the soil to form various nitrate compounds, such as Calcium Nitrate, Potassium Nitrate, etc. Thus atmospheric nitrogen mixes with the soil (2) & (5).
2HNO₃ + CaCO₃ (Calcium carbonate) → Ca(NO₃)₂ + CO₂ (Calcium nitrate)
Biological process
The role of various soil microorganisms in fixing atmospheric nitrogen in a natural way is significant. There are three main types of microorganisms involved in this process. The biological process was discovered by Hermann Hellriegel and Martinus Beijerinck.
1. Symbiotic bacteria
Leguminous plants such as peas, soybeans, cloves, green peas, lentils, chickpeas, Asian pigeonwings, etc. have a type of nodules at their roots. The Rhizobium bacteria living in these nodules participate in the fixation of atmospheric nitrogen. These bacteria absorb atmospheric nitrogen directly. After these bacteria absorb nitrogen directly from the atmosphere, some of the nitrogen is supplied to the host plant and the rest forms nitrogen compounds in their bodies. These bacteria supply ammonia and amino acids to the host plants and survive by taking organic matter from host plants. When the plant dies, the bacteria die and the nitrogen present in the body of the bacteria is released into the soil.
2. Free-living bacteria
There are some nitrogen-fixing-free living bacteria present in the environment. The nitrogen-fixing bacteria are Azotobacter (aerobic bacteria) and Clostridium (anaerobic bacteria). These bacteria fix nitrogen in the soil as ammonia. This process is accelerated in the presence of molybdenum. Again the presence of synthetic chemical fertilizers increases the activity of Azotobacter. As a result, the amount of nitrogen in the soil increases.
3. Blue-green algae
Some blue-green algae participate in nitrogen fixation. About 30 to 40 species of blue-green algae are capable of nitrogen fixation. The most notable blue-green algae species are Anabaena, Nostoc, Calothrix, etc. These types of algae absorb nitrogen directly from the atmosphere and form nitrogen compounds in their bodies. The nitrogen present in their body of bacteria and algae is finally released into the soil. The blue-green algae are actually a type of cyanobacteria.
Apart from these microorganisms, purple bacteria, Rhodospirillium, some photosynthetic bacteria, and some bacteria like Pseudomonas also can fix the atmospheric nitrogen in the soil. Besides, some of the nitrogen comes into the soil through synthetic chemical fertilizers used in agriculture such as ammonium sulfate, calcium nitrate, etc. At present, the application of chemical fertilizers also produces about 30 X 10⁶ tons of nitrogen per year. The amount of nitrogen is gradually increased day by day.
Biological methods are the most important in nitrogen fixation. About 54 X 10⁶ metric tons of nitrogen per year is fixated or stabilized by these methods. The biological fixation adds about 140 million metric tons of nitrogen to ecosystems every year (2) & (4).
Importance of nitrogen fixation
Nitrogen fixation is a very important process of the nitrogen cycle. In this process, various forms of nitrogen converted and mixed with soil. The importance of nitrogen fixation in nature is immense.
- Plants absorb nitrogen when it is converted to ammonia and nitrate. Plants absorb this converted nitrogen from soil through their roots. The nitrogen then enters the other parts of the plant. This nitrogen is used to form plant tissues and cells. As a result, the plants grow. Thus nitrogen fixation plays an important role in plant growth.
- Nitrogen fixation helps to increase the fertility of agricultural land. Nitrogen is a very important element of agricultural land. It increases soil fertility. Therefore after cultivating paddy on agricultural land, farmers cultivate peas, lentils, etc. The roots of these plants contain a type of bacteria that absorbs atmospheric nitrogen directly. As a result soil fertility increases.
- There are various nitrogen-fixing bacteria that participate in nitrogen fixation. These nitrogen-fixing bacteria increase the water holding capacity of the soil (1) & (2).
Nitrogen fixation has a significant impact on the environment. Atmospheric nitrogen is stabilized or fixed in the soil by various bacteria and returns to the atmosphere in different steps. The nitrogen fixation process converts inert nitrogen to active nitrogen. This nitrogen is used in various cases. So nitrogen fixation is very important for all organisms.
Written By: Manisha Bharati
