Introduction
Nucleic acid is a polymer-type chemical that regulates the various biological functions of an organism. It is basically a polymer of nucleotides. Three types of components are found after hydrolysis of nucleic acid namely pentose sugar, nitrogenous bases, and phosphoric acid. Guanine is a type of nitrogenous base (5).
These nucleic acids are the most important biological molecules they contain two types of bases namely purines and pyrimidines. They are composed of nitrogen, carbon, hydrogen, and oxygen making up these single-ringed or two-ringed bases (4).
Among these two bases of nucleic acids, purines are composed of two nitrogenous bases. Guanine is one of them. It is bound to cytosine (in the case of deoxyribonucleic acid) or to uracil (in the case of ribonucleic acid) by three hydrogen bonds. This nitrogenous base is very important. So its structure, features, and function are described below (3) & (6).
Guanine
The guanine word comes from the Spanish word “Guano”. A nitrogenous base, which exists as the material of the RNA of a cell and the nucleotides of DNA is known as guanine. This base is revealed by G. Its chemical symbol is C₅H₅N₅O. Its nucleoside is called guanosine. Some of the features of this nitrogenous base are discussed below (6).
It was first discovered in 1846. But this base was isolated in 1891 from nucleic acids. The sugar ribose, deoxyribose, nucleotides guanylic acid, and deoxyguanylic acid are combined with this nucleobase. It derives from a hydride of a 9H-purine (1) & (2).
Properties
- It is a purine base.
- This base is a component of nucleotides made up of nucleic acids.
- It is a primary component of other physiologically important molecules such as guanosine-5- triphosphate.
- This nucleobase is insoluble in water. However, it can dissolve acids and alkalis.
- Its molar mass is about 151.13 gm/mol.
- The melting point of this purine base is 360°
- It can be synthesized from amino acids in the body.
- The base is an important component of the nucleic acid metabolism of organisms.
- It binds to cytosine in the structure of DNA.
- It is a colorless and crystalline substance (4) & (7).
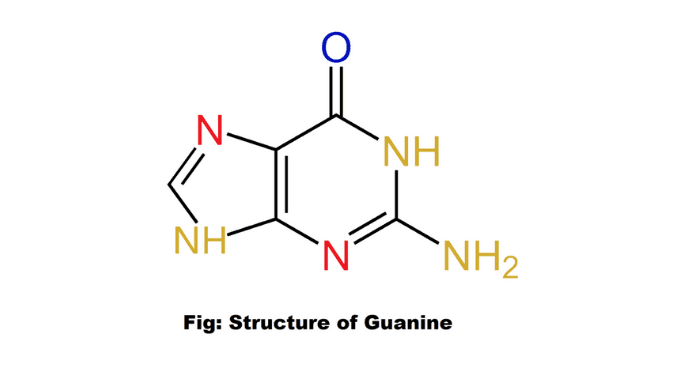
Structure
Guanine (G) is a purine nitrogenous base with the formula C₅H₅N₅O. It is a double helix shape. This base is one of four chemical bases in DNA. The structure of this nitrogenous base (2-amino-6-hydroxypurine) is a heterocyclic purine ring consisting of a system of two connected rings: one ring is pyrimidine and the other ring is imidazole.
The heterocyclic ring of this purine base is flattened with a few connected double bonds. This nucleobase is paired with cytosine in DNA. It is attached to a six-carbon pyrimidine ring with a five-carbon imidazole ring, forming a system stabilized by double bonds. Three hydrogen bonds are formed between guanine and cytosine (5).
It is linked to 1- carbon of two types of pentose sugars by glycosidic bonds. There are two functional groups present in this nucleobase. These are an amino group in C-2 and a carbonyl group in C-6. In both DNA and RNA, this nitrogenous base is paired with pyrimidine and cytosine. It is found in two tautomeric forms such as the major keto form and the rare enol form (1) & (7).
Functions
This nitrogenous base plays an important role its functions are also associated with GMP, GDP, and GTP.
- It is the building block of DNA and RNA.
- This nitrogenous base acts as a component of nucleic acids and various nucleotides and nucleosides.
- Like ATP, it acts as a source of energy in protein synthesis as well as gluconeogenesis.
- It forms the genetic code with the other three nucleus bases adenine, cytosine, and thymine.
- The gamma phosphate group of GTP may be converted to adenosine 5-triphosphate for ATP formation. GTP is a purine nucleoside triphosphate.
- It plays a vital role in RNA synthesis as well as nucleic bases for protein synthesis.
- GMP is the most stable form of nucleotide that contains it. Through hydrolysis, GMP cyclic forms GMP, which is the second receptor during intercellular signaling in the translation pathway.
- GTP, which contains this purine base, acts as a free energy source for protein biosynthesis in ribosomes.
- In neurons of the hippocampus, the activity of voltage-gated sodium channels is regulated by hydrolysis of GTP to GDP.
- It also acts as the second messenger of GTP.
- This nitrogenous base forms complementary base pairs with cytosine in both DNA and RNA (5) & (6).
Q&A
1. Which mRNA nucleotide is complementary to guanine?
There are four nucleotides in mRNA, viz. adenine (A), guanine (G), cytosine (C), and uracil (U). The nucleotide bases are linked by a hydrogen bond on opposite strands of DNA or double strands of RNA. Both DNA and RNA have specific base-pairing rules. According to Chargaff’s rules, cytosine is complementary to guanine.
2. What is guanine?
Guanine is one of the nitrogenous bases in nucleotides. There are two types of nitrogenous bases in nucleotides, purine, and pyrimidine. It is a purine base made up of two rings of nitrogen and carbon atoms. The nucleobase is synthesized during the synthesis of purine bases via IMP (Inosine monophosphate). Since it is a part of nucleic acid, it is freely available as an intermediate product of metabolism (4).
3. If a DNA sample were composed of 10% thymine, what would be the percentage of guanine?
Based on Chargaff’s rules, the amount of adenine (A) in the DNA of an organism and the amount of thymine (T) is the same. That is, A = T.
Similarly, the amount of guanine (G) and cytosine (C) in DNA is also the same. That is, C= G.
So if a DNA sample were composed of 10% thymine, adenine also be 10% (according to Chargaff’s rules).
The remaining 80% of DNA is made up of guanine and cytosine. The amount of guanine and cytosine is equal, which means, guanine is 40% and cytosine is also 40%. So If DNA samples were composed of 10% thymine, the percentage of guanine is 40%.
4. What does guanine pair with 200 if adenine makes up 20% of the bases in a DNA double helix?
If DNA is 20% of adenine then the amount of thymine is also 20%, because the amount of adenine and thymine is equal.
20% + 20% = 40% (According to Chargaff’s law).
From 100% DNA bases subtract 40% and the rest is 60%. Then divide this 60% by 2. And 30% of guanine and 30% of cytosine are obtained because their amounts are equal in the DNA strand.
5. What percent of the bases are guanine?
According to Chargaff’s rules, the DNA of all organisms should have a 1:1 ratio (base Pair Rule) of purine bases (for the DNA cytosine, thymine, and for the RNA uracil) and pyrimidine bases (guanine and adenine for RNA and DNA).
The amount of guanine (G) is equal to cytosine (C) and the amount of adenine (A) is equal to thymine (T). That is- A=T & C= G.
Cytosine pairs with guanine and adenine pairs with thymine. If DNA is 20% of adenine then the amount of thymine is also 20%. From 100% DNA bases subtract 40%. Then the rest is 60% is divided by 2 and 30% of guanine and 30% of cytosine are obtained because their amounts are equal in the DNA strand. So 30% of the bases are guanine.
Written By: Manisha Bharati
Reference
1. L. Dutta. Inorganic Chemistry: Chemical Elements and their Compounds. Part- II. The New Book Stall, Kolkata. Chapter: Chemistry of Nucleic Acids. Page No: 256 to 271 & Chapter: Biosynthesis of Nucleic Acids. Page No: 565 to 570.
