Definition
Cytochromes are redox-active proteins containing a heme, with the central Fe (iron) atom at its core, as a cofactor. They are involved in the electron transport chain and redox catalysis.
Cytochromes are classified according to the type of heme and its mode of binding. They are found in both animals and plants and contain heme-like prosthetic groups. Cytochromes are a family of flavoproteins widely distributed in the eukaryotic phyla.
They work as electron transfer agents. The active site of cytochrome is iron (Fe). They are present in Chloroplast and mitochondria.
Types of Cytochromes
There are three types of cytochrome according to the prosthetic group
Groups Prosthetic group
1. Cyto a Heme A
2. Cyto b Heme B
3. Cyto c Heme C (covalently bound heme B)
Electron flow system in the respiratory chain
When electrons are transported along the respiratory chain in a high amount of energy released, ATP molecules are synthesized at the following three sites.
1. Transfer electrons from NADH to ubiquinone via flavoproteins
2. It transfers electrons from Cyto b to Cyto. C
3. Transfer of electrons Cyto a to Cyto a3
Electron flow into cytochromes
Cyto b to Cyto c → Cyto a to O2
Cytochrome a
In Cytochrome a, heme group has a formyl group replacing a methyl group. It is capable of binding oxygen and reducing it. And responsible for the severe toxicity of cyanide. It participates in the electron transfer process.
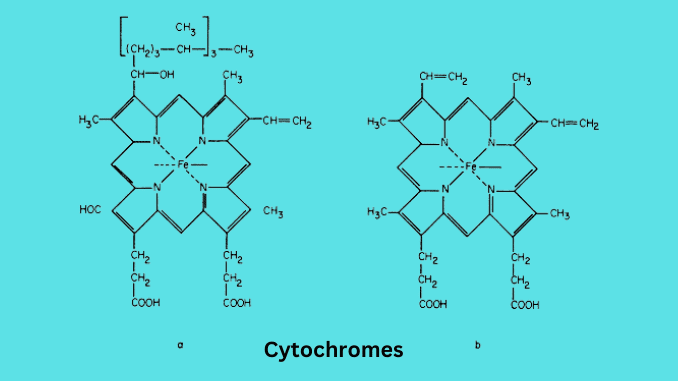
Cytochrome b
Cytochrome b consists of the heme b prosthetic group. It contains two heme groups. Heme b is similar to hemoglobin and myoglobin but protein is not attached to heme.
Cytochrome c
Cytochrome c is made with heme c and protein is bound with covalent linkage. The heme group has a polypeptide chain attached. The chain consists of variable no. Of amino acids.
A nitrogen atom of the histidine segment and a sulfur atom of the methionine segment of this chain are coordination sites of the iron atom.
Cytochrome c reacts indirectly by the electron transfer mechanism. It is an example of electron transferase. Electrons released from the Oxidation of carbohydrates are transported to dioxygen in mitochondrial cells.
Cytochrome P450
Cytochrome P450 is also called mono-oxygenase. In Cytochrome P450, “P” stands for pigment and 450 is the wavelength of light in nanometers (nm). Cytochromes work as oxidase and absorb a maximum of 450 nm radiation. Cyto P450 is found in plants, animals, and bacteria. It is abundant and present in the endoplasmic reticulum of liver cells and is also seen in the adrenal gland.
It is the heme-containing enzyme that catalyzes the insertion of oxygen into substrates.
About Cytochrome
- Cytochromes are hemoproteins taking part in redox reactions by reversible transitions between the
- Fe ++ and Fe +++ states. Cytochromes are members of electron transfer chains (respiratory chain, photosynthesis)and participate in enzymatic reactions (e.g. cytochrome P-450).
- They occur in many variants in almost all organisms (except in a number of anaerobic bacteria) Hemes are also present in other enzymes, e g catalase, and peroxidases.
- Cytochromes are grouped by the substitutions at the porphyrin ring and by the fifth and sixth ligands of the Fe atom (in addition to the 4 ligands provided by the porphyrin ring). The ring substitutions of cytochromes b are identical with protoporphyrin IX and with heme in hemoglobin.
- Cytochromes d is a chlorine with a reduced ring c. The individual cytochromes are named by the peak wavelength of the light absorption band alpha.
- Many cytochromes are transmembrane proteins with several membrane Spanning helices In some cases 2 hemes are connected with a single protein Chain Other cytochromes are peripheral Proteins that act as electron shuttles.
References
Biochemical Pathways: An atlas of biochemistry and molecular biology
4th edition biochemistry Donald Voet & Judith G. Voet
Written By: Richa Pachori
