Introduction
Weathering is a process of breaking rocks and complex matter in the environment into simple substances under the influence of the atmosphere. There are three types of weathering. These are physical weathering or mechanical weathering, chemical weathering, and biological weathering. Below is a discussion of chemical weathering.
The physio-chemical conditions of the rock layer are different at the time of formation and at the time of exposure to the earth’s atmosphere. The effect of these changes on the bedrock is weathering. For example, when a granite bedrock is formed as an igneous rock, the heat, and the pressure that builds up change as the bedrock is exposed.
Weathering is a significant process in the environment. Through this process, the soil is formed, decomposed, and regenerated. Soil is formed from rocks through natural and chemical changes such as weathering etc. in the presence of organic matter. There are different mechanisms of weathering. One of them is chemical weathering (1) & (3).
What is chemical weathering?
If the rock decomposes in a chemical reaction under the influence of oxygen, carbon dioxide, water, and water vapor, it is called chemical weathering (1).
-
Affected areas
Chemical weathering is particularly important everywhere except in the desert and Polar Regions. Its prevalence is highest in equatorial warm and humid and tropical humid climates. Chemical weathering is important in warm and humid temperate regions (2).
Interesting facts about chemical weathering
- This type of weathering is most effective in humid equatorial, monsoon, etc. climates.
- In this case, different gasses in the atmosphere and water play a major role.
- When rock-intermediate minerals come into contact with gas or water, rapid chemical weathering occurs, and the effects can be seen relatively quickly.
- This process is fairly silent. The temperature rises during the reaction.
- In this process, the primary minerals in the rock are easily eroded into secondary minerals.
- In this weathering process, the rock is broken down through chemical reactions.
- During the reaction, the volume of the mineral decreases and increases.
- Water plays a major role in this type of weathering.
- Among the regions affected by chemical weathering are tropically warm and humid climates (1) & (4).
Favorable climate and environment
This type of weathering mainly occurs due to water and water vapor. For this reason, warm-humid climates are particularly favorable for chemical weathering. That is why chemical weathering is more effective in equatorial climates and tropical climates (4).
Types of chemical weathering
1. Carbonation
Carbonization is one of the chemical weathering methods. The chemical reaction of natural carbon dioxide (CO₂) with various minerals causes the rock to disintegrate, and the original minerals are easily eroded into new minerals. This process is called carbonization.
As rainwater falls through the atmosphere to the surface, it combines with carbon dioxide in the atmosphere to form carbonic acid (H₂CO₃). As the carbonic acid precipitates on the limestone, the calcium carbonate Ca(HCO₃) is easily dissolved and removed. In the intercalated areas, the limestone is disintegrated and eroded continuously to form different landforms (1) & (2).
Formula
- H₂O + CO₂ = H₂OCO₃
- H₂CO₃ + CaCO₃ = Ca(HCO₃)₂
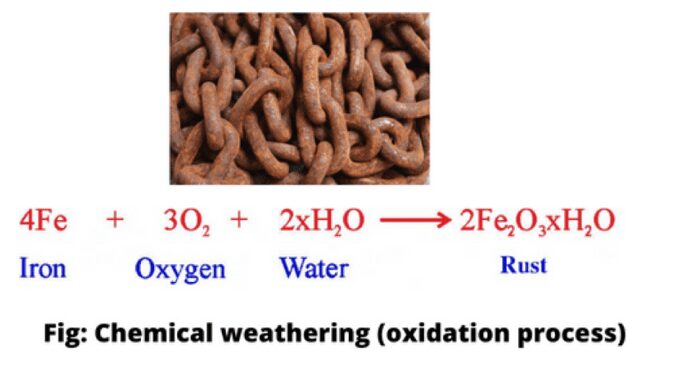
2. Oxidation
When oxygen is combined with minerals in a chemical process, it is called oxidation. It reacts very easily with iron. Oxidation of solid iron changes the parent mineral from ferrous oxide to ferric oxide to form yellow or brown-colored limonite which breaks down easily.
Formula
Fe₃O₄ + H₂O + O₂ → 2Fe₂O₃ 3H₂O
Oxidation is a process in which an atom or ion loses an electron to gain an increase in positive charge and a decrease in negative charge. Oxygen dissolved in water is the most universal oxidizing agent. This reaction can be reversed by gaining an electron through a reduction process. Various materials such as iron, titanium, manganese, or Sulphur, can be subjected to the oxidation process to form oxides or hydroxides (2) & (5).
Formula
4Fe²⁺ + 3O₂ → 2Fe₂O₃
3. Hydration
When water is added in a chemical process, the strong bond between the molecules of the mineral is destroyed, making the new mineral more flexible than the original mineral and increasing in volume. This is called hydration.
Some minerals have a remarkable ability to absorb water in their crystalline structure through a biphasic reaction. This process is known as the hydration process. Some minerals swell and expand by absorbing water from the air in a chemical process. As a result, strein is created in the rock. Anhydrite becomes Gypsum in this process (3) & (5).
Formula
C₂SO₄ + 2H₂O → CaSO₄, 2H₂O
4. Hydrolysis
The weathering that takes place when water is combined with the molecular structure of the mineral is called hydrolysis.
In this process, water is broken into hydrogen ions and hydroxyl ions and the resulting hydroxyl ions change through chemical reactions in the mineral. The feldspar rocks are separated into these types. In addition to its role as a solvent, water is capable of reacting directly through the hydrolysis process.
In this process, the cation is removed by H⁺ ions and this released cation combines with the hydroxyl ion. Thus, by chemical weathering, the silicate mineral albite is weathered into the clay mineral kaolinite.
Formula
4NaALSi₃O₈+ 6H₂O→ Al₄Si₄O₁₀(OH)₈ + 8SiO₂ + 4Na⁺ + 4OH⁻
Some silicon remains in kaolinite and sodium is removed from the solution. This reaction produces hydroxyl ions and can render surface water alkaline. Although in most environments this water is neutral or slightly acidic (2).
5. Solution
The solution is one of the various methods of chemical weathering. A few minerals like rock salt, gypsum, etc. dissolve in contact with water and lose their own shape, this particular process is called a solution.
Although few minerals are soluble in water, solution indirectly plays a prominent role in weathering as water dissolves and removes various weathered products. This is the simplest process in which minerals are dissociated and water acts as a solvent in this dissociation. The following shows how quartz dissolves.
Formula
SiO₂ + 2H₂O → Si(OH)₄
The solubility of a mineral is the extent to which it will dissolve in water, it is usually expressed in ppm or parts per million. Some minerals like halite are highly soluble but quartz is very sparingly soluble. It is very slowly soluble in water. Solubility is affected by the temperature and pH of the environment.
As the water associated with the mineral gradually becomes saturated with dissolved substances, the rate of dissolution decreases. It must then be dissolved in non-saturated water. Only then the process of increasing the dissolution rate of minerals will be effective (1).
Chemical weathering examples
- When carbon dioxide and water are combined with calcium carbonate or limestone, it turns into calcium bicarbonate in a chemical reaction and starts to decay easily. This is an example of chemical weathering (5).
Formula
CaCO₃+ H₂O+ CO₂ → Ca(HCO₃)₂
- Iron products easily rust and deteriorate. This is because the oxygen in the air combines with the iron to form brown-colored limonite which breaks down easily. Amphibole and pyroxene are easily altered in this process (2).
Formula
MgFe₂SiO₄+ 2HOH→ Mg(OH)+ H₂SiO₄+ FeO
Chemical weathering definition
As a result of the reaction of elements like oxygen, carbon dioxide, water vapor in the atmosphere, and the minerals in the rock, the hardness of the rock decreases and the rock is weathered. Because of this reaction, the primary minerals in the rock are easily eroded into secondary mineral particles. Thus, when rocks are weathered by chemical reactions, it is called chemical weathering (1).
Examples of chemical weathering
- Some minerals swell and expand by chemically absorbing water from the air. This results in cracks in the rock. Anhydrite turns into gypsum through a chemical weathering process.
Ca₂SO₄+ 2H₂O → CaSO₄, 2H₂O
In this process, iron is combined with water to form limonite.
2Fe₂O₃+ 3H₂O = 2Fe₂O₃, 3H₂O
- In the hydrolysis process, the metal cation is removed by H⁺ ions and this released cation combines with the hydroxyl ion. Thus, by chemical weathering, the silicate mineral albite is weathered into the clay mineral kaolinite (2).
4NaALSi₃O₈+ 6H₂O→ Al₄Si₄O₁₀(OH)₈ + 8SiO₂ + 4Na⁺ + 4OH⁻
Q&A
1. What is chemical weathering?
The elements like oxygen, carbon dioxide, water vapor, etc. in the atmosphere react with the minerals in the rock in different ways and destroy the hardness of rock. As a result, the rock is separated. Because of this reaction, the primary minerals in the rock are easily weathered into secondary minerals. Thus when the rock is broken down by chemical reactions, it is called chemical weathering.
2. What two factors speed up rates of chemical reaction and weathering in rocks and soils?
Warm temperatures and the presence of moisture are the two factors that speed up rates of chemical reaction and weathering in rocks and soils.
3. Which of the following is an example of chemical weathering?
Rust formation is an example, which occurs in the oxidation process. It is formed when oxygen reacts with iron to form rust.
4. What causes chemical weathering
It causes rainwater to react with mineral grains in rocks to form new minerals and soluble salts. These reactions occur especially when the water is slightly acidic.
5. Which of the following is/are most susceptible to chemical weathering by dissolution?
Calcite is most susceptible to chemical weathering by dissolution.
Written By: Manisha Bharati
Reference
1. Savindra Singh. Geomorphology. Pravalika Publications, Allahabad. Chapter 14: Weathering and Mass movement. Page No: 247- 266.
